Abstract
The healthcare burden of cardiovascular diseases remains a major issue worldwide. Understanding the underlying mechanisms and improving identification of people with a higher risk profile of systemic vascular disease through noninvasive examinations is crucial. In ophthalmology, retinal vascular network imaging is simple and noninvasive and can provide in vivo information of the microstructure and vascular health. For more than 10 years, different research teams have been working on developing software to enable automatic analysis of the retinal vascular network from different imaging techniques (retinal fundus photographs, OCT angiography, adaptive optics, etc.) and to provide a description of the geometric characteristics of its arterial and venous components. Thus, the structure of retinal vessels could be considered a witness of the systemic vascular status. A new approach called “oculomics” using retinal image datasets and artificial intelligence algorithms recently increased the interest in retinal microvascular biomarkers. Despite the large volume of associated research, the role of retinal biomarkers in the screening, monitoring, or prediction of systemic vascular disease remains uncertain. A PubMed search was conducted until August 2022 and yielded relevant peer-reviewed articles based on a set of inclusion criteria. This literature review is intended to summarize the state of the art in oculomics and cardiovascular disease research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Literature on artificial intelligence, retinal imaging and cardiovascular disease assessment is constantly growing. New tools are available to extract microvascular information from retinal imaging to screen and predict patients’ cardiovascular status. |
This literature review is intended to summarize the state of the art in oculomics and cardiovascular disease research. |
What was learned from the study? |
Oculomics is a fantastic tool to bring incremental personalized value to conventional cardiovascular risk assessment scores. |
Oculomics showed promising results, and it now needs to be applied to real-world healthcare cardiovascular workflows. |
Introduction
Over the past 2 decades, interest in retinal vascular imaging has been increasing. Technological developments in modalities such as retinal fundus photography, optical coherence tomography–angiography (OCT-A) or adaptive optics have made it possible to derive accurate retinal vascular metrics. For example, data on vessel calibre, tortuosity, branching angle and retinal fractal dimension can be extracted from fundus photographs using semi-automated analysis software with reproducible algorithms [1,2,3]. With OCT-A, it is possible to describe the retinal vascular network at capillary level in different plexuses. Effective quantitative retinal vascular metrics, such as vessel density, vessel perfusion and flow index, are available with OCT-A devices [4,5,6]. These fundus photograph and OCT-A retinal vascular metrics were shown to be correlated with the detection, severity and progression of various clinical eye diseases (e.g., diabetic retinopathy, vascular occlusion, glaucoma) [7,8,9,10,11]. Fundus and OCT-A features were associated not only with vascular ocular disorders, but also with systemic vascular diseases [12]. Indeed, it is recognized that small-vessel or microvascular pathology plays a major role in processes leading to the development of cardiovascular disease (CVD) and its risk factors [13,14,15]. However, the microcirculation has been difficult to access, and thus robust microvascular biomarkers have yet to be developed. The retinal vasculature, which can be accessed noninvasively, represents a unique biological model for the study of microvascular abnormalities and pathology associated with cardiovascular disease [16,17,18]. Numerous studies have investigated the relationship between retinal microvascular abnormalities and systemic system. Hence, impaired retinal vascular network patterns were associated with increased cardiovascular risk score assessment [18, 19], cardiovascular mortality [21] and cardiovascular risk factors [22]. The field of ocular biomarkers of systemic disease is now conceptualized as “oculomics” research [23, 24].
However, recent rapid developments in artificial intelligence (AI) in medical imaging hold promise for improved screening, diagnostics and community healthcare [25]. In the field of retinal vascular imaging, AI techniques have found a particularly good fit. Initially, researchers focused on high-incidence ocular diseases such as diabetic retinopathy [26, 27], age-related macular degeneration [28] or glaucoma [29, 30]. Cardiovascular risk stratification was recently presented as an accessible outcome for AI algorithms and retinal vascular imaging. Indeed, historical prediction models for CVDs such as the Framingham risk score [31] and the recently updated SCORE2 [32, 33] in the general population may have some limitations in specific ethnic groups and intermediate risk profile patients [34, 35].
Hence, researchers investigated automatic image analysis algorithms to highlight markers of retinal vascular health to confirm previous findings on the association between feature-based retinal microvasculature parameters and cardiovascular status. Machine learning (ML) and deep learning (DL) showed huge potential for automatic analysis and quantification of retinal vascular biomarkers to predict cardiovascular risk factors and vascular systemic events [36, 37].
The scientific literature on this topic is constantly growing. In the present literature review, we summarize the recent developments in AI for retinal vascular imaging (retinal fundus photographs and OCT-A) and cardiovascular profile evaluation. The aim of this review is to critically evaluate the state of the art in “oculomics” and cardiovascular disease research.
Techniques in AI to Extract Retinal Microvascular Parameters
Machine Learning
ML is one of the many subsets of AI and refers to creating programmes based on data as opposed to programming rules. ML has its roots in large datasets and identifies interaction patterns among variables [38]. These techniques can discover previously unknown associations and thereby generate novel hypotheses. In medicine, ML is widely used to build automated clinical decision systems. Most ML approaches fall into two main categories: supervised and unsupervised methods [39]. Unsupervised learning does not require labeled data. It aims to identify hidden patterns present in data and is often used in data exploration and generation of novel hypotheses. Supervised learning starts with the goal of predicting a known output or target. It focuses on classification, which involves choosing among subgroups to best describe a new data instance, and on prediction, which involves estimating an unknown parameter. The computer approximates what a trained physician is already capable of doing. This task is achieved through the extraction of meaningful and robust features (invariance to illumination, translations, scale, etc.), which are known as engineered or hand-crafted features, that require a huge amount of time or heuristic approaches [40].
Deep Learning
DL is a specialized subset of ML that imitates the neural structure of the central nervous system by creating artificial neural networks (ANNs). An ANN is a computing system based on a network of units called “artificial neurons” organized into layers. Layers of neurons perform transformations of the signal as it travels from the input (first) layer to the output (last) layer. DL is presented when deep neural networks (DNNs) form the basic architecture of AI algorithms. A DNN is an ANN with multiple intermediate layers positioned between the input and output layers. It allows each level to learn to transform its input signal into a gradually more complex and higher-level representation, making them more efficient at learning. A key benefit of DNNs is that their performance was shown to continuously improve with the size of the training dataset. The DL architecture found to be most suitable for retinal vascular imaging data is that of convolutional neural networks (CNNs). CNNs encode connectivity patterns between neurons that mimicked the organization of the mammalian visual cortex. Trained with large annotated datasets, CNNs essentially allowed computers to start recognizing visual patterns and are primarily responsible for the recent resurgence and overwhelming interest in AI within the field of retinal imaging [41].
Publicly Available Dataset for Retinal Imaging
Retinal imaging analysis with AI was boosted by the availability of large, real-world images datasets. Recently, robust retinal imaging datasets (i.e., MESSIDOR, STARE project, DRIVE, E-ophtha and EyePACS) with accurate labelling, high image quality and operable format were used by different research teams [27, 42,43,44,45,46]. A recent review highlighted 94 open-access ophthalmology datasets with completely available access [47]. Khan et al. were able to access 507,724 images from at least 122,364 individuals. The most common types of retinal images were fundus photographs followed by OCT and OCT-A images. Researchers could access images of healthy, myopic, hypertensive and diabetic eyes with basic demographic data (age and sex) from different ethnic groups (Asia, North Africa, Middle East, Europe, North America). These publicly available datasets allow researchers to analyse images from heterogeneous populations and from different devices and then to refine their algorithms using these external datasets. Moreover, various imaging modalities have been available with different programmes, for example, screening programmes in primary, secondary and tertiary centres, which reinforces the generalization of AI algorithms.
Fundus Image Processing from Semi-automated to Automated Methods
Traditional Semi-automated Feature-based Image Analysis Methods
Since the late 1940s, ophthalmologists have been interested in the association between subjective and qualitative retinal vascular signs on fundus photographs (e.g., retinal haemorrhages, exudates, cotton wool spots, diffuse or focal reduction in vascular calibre) and systemic vascular diseases (hypertension and arteriosclerosis) [48]. The ability to image the retina and its vasculature was a turning point in this approach. Parr et al. were among the pioneers in developing objective and reproducible quantitative measurements of retinal vascular calibre from fundus photographs [49]. To this end, three software programmes are more commonly used, namely, Integrative Vessel Analysis (IVAN), Singapore I Vessel Assessment (SIVA) and Vascular Assessment and Measurement Platform for Images of the Retina (VAMPIRE). Retinal microvascular parameters are measured based on a semi-automated detection of retinal arterioles, venules and optic nerve head. Various quantitative retinal vascular parameters can be computed objectively over up to two disc diameters such as retinal calibre expressed as central retinal arteriolar equivalent (CRAE), central retinal venular equivalent (CRVE), arteriole-to-venule diameter ratio (AVR)[50,51,52], tortuosity [53, 54], branching angle [55] and fractal dimension [2, 56,57,58,59]. These software programmes were found to be highly repeatable and reproducible [52, 60].
For more than 20 years, numerous research teams have been working on the associations between retinal microvascular characteristics extracted from these softwares and systemic vascular diseases. Retinal microvascular abnormalities (reduced arteriolar and wider venular calibre, increased tortuosity, suboptimal retinal vascular network) were associated with hypertension [18, 61,62,63,64,65], cardiovascular mortality [21, 56, 66, 67], ischaemic stroke [59, 68, 69] and increased cardiovascular risk score [19]. Furthermore, theses associations were confirmed in multiple large population-based studies from different ethnic groups, including European [19, 70, 71], Australian [72], North American [60, 73] and Asian cohorts [74].
Nevertheless, these semi-automated software programmes presented several limitations, which led to a drive for new retinal image technology. Indeed, these original approaches were extremely time consuming for large fundus datasets; moreover, the agreement or inter-changeability between different software programmes was debatable [75,76,77]. Another limitation was that the software depends greatly on the architecture and definitions of the retinal microvasculature. The definitions of the retinal image parameters were determined in the early studies based on either clinical observation (e.g., CRAE and AVR to represent retinal arteriolar narrowing [51]) or the hypothesis of an optimal state in the vascular structure based on physiological theories (e.g., branching angles and junctional exponent to represent Murray’s law) [78]. Even though the feature-based semi-automated approach demonstrated a close link between morphological features of the retinal vasculature and systematic diseases, it is possible that a vast amount of undefined information remains hidden in the retina. In that respect, increasing attention was given to automated fundus processing with AI techniques aiming to detect retinal biomarkers for cardiovascular risk assessment.
Automated Image Processing with AI Techniques
To reduce the involvement of the operators and move from semi-automated to fully automated image processing, several DL-based algorithms have emerged [79,80,81] for either segmenting biomarkers (veins, fovea) or grading the image quality [82]. Since most of the information is supplied by the retinal vessels in oculomics, automated vessel segmentation was the main focus of the research community, with most publications concentrating on this area as compared with other retinal biomarkers (optic disk, fovea [83, 84]). Most of the recent architectures are based on the UNet architecture [85] originally published in 2015, with some clever adaptations in the network design [86, 87], adding dense blocks [88], squeeze-and-excitation blocks [89], spatial attention modules [90], spatial positional attention modules [91] or specifically designed losses [92]. Going further, recent architectures are leveraging the image transformer architectures [93] to propose a patch convolution attention-based transformer UNet (PCAT)-UNet) [94]. However, these UNet-like or transformer architectures are heavier (in terms of parameters), harder to train and greedy in terms of data, and the outputs are more complex to interpret.
A good example of automated image processing software is the QUARTZ software. It went from automated retinal vessel morphometry segmentation [95], differentiation of arterioles and venules [96] and retinal geometric feature quantification [97] to epidemiological studies [98].
Galtran et al. [99] recently showed, through a detailed evaluation, that the performance of a simple downgraded UNet was equal to that of more complex models (several orders of magnitude in terms of model parameters) for vessel segmentation on fundus and OCT-A images. They also highlighted that these systems still exhibited a decreased performance across databases, leaving room for further improvements before application in daily clinical routine.
Cheung et al. developed a DL algorithm for automated measurements of retinal-vessel calibre from retinal photographs (SIVA-DLS) [37]. The ground truth of these models was retinal-vessel calibre measured with the software SIVA (named “SIVA-human”). Agreement between SIVA-human and SIVA-DLS regarding retinal-vessel calibre was high (ICCs ranging from 0.82 to 0.95). The authors validated a large-scale automated model to replace semi-automatic software that was previously time consuming (SIVA-human, approximately 25 min for each grading fundus image), expensive and not suited for large datasets.
Finally, the DL approach has been shown to perform better than the feature-based approach; however, inherent to the former method are the difficulties in interpreting its models, known as “the black box problem” [100]. Moreover, these DL models, like any data-based model, require a large and representative (multi-vendor, multi-centric, multi-ethnicity) sample of annotated data, which also impedes the adoption of these systems in clinical settings.
Cardiovascular Risk Assessment Using AI-derived Microvascular Parameters
Prediction of Cardiovascular Risk Factors
One of the first applications of AI in this area of research was the prediction of classic cardiovascular risk factors based on retinal fundus images alone. Poplin et al. designed a CNN model to predict multiple cardiovascular risk factors based on two datasets (UK Biobank and EyePACS) [36]. The results of the DL algorithm were relevant notably for the prediction of age [mean absolute error (MAE), years (95% CI) 3.26 (3.22, 3.31)], gender [area under the curve (AUC), (95% CI) 0.97 (0.96, 0.97)], smoking status (current smoker) [AUC, (95% CI) 0.71 (0.70, 0.73)] and systolic blood pressure [MAE, mmHg (95% CI) 11.35 (11.18, 11.51)]. Kim et al. also found a highly accurate prediction for age with the CNN ResNet-152 algorithm based on 24,366 fundus images [MAE, years (95% CI) 3.06 (3.03–3.09)] [101]. Interestingly, the authors reported that differences between retinal-fundus-predicted age and chronological age were higher after 60 years of age and in patients with systemic vascular disease (hypertension and diabetes mellitus). With a smaller dataset (1222 fundus photographs), Zhang et al. produced accurate prediction rates for cardiovascular risk factors. Cheung et al. found associations of central retinal arteriolar equivalent with age, gender, mean arterial blood pressure (MABP), body mass index and total cholesterol as well as associations of central retinal venular equivalent with gender, MABP, body mass index, glycated haemoglobin and current smoking were similar between the automated model (SIVA-DLS) and the semi-automatic software (SIVA-human) [37]. Another strategy was presented by Arnould et al., who focused not on the raw retinal images but on quantitative geometric metrics obtained from the Singapore “I” Vessel Assessment (SIVA) and Angioplex (version 10; Carl Zeiss Meditec AG) software [102]. They trained supervised ML algorithms based on solo and combined (retinal fundus and OCT-A) vascular quantitative geometric metrics to predict age, diabetes mellitus history and hypertension history.
Finally, one of the major cardiovascular risk factors is ageing [103]. Original approach was to assess physiological age based on retinal fundus. This new feature (physiological age estimation based on fundus = RetiAGE) could be used as a cardiovascular risk factors in future study [104].
Prediction of CVD Biomarkers
For evaluation of the cardiovascular risk profile of patients, the American College of Cardiology/American Heart Association (ACC/AHA) recommend analysing the Framingham Risk score 10-year cardiovascular disease calculation (FRS) [105] and more recently the Pooled Cohort Equations (PCE) [106]. However, these calculations may prove to be unreliable in daily clinical routine in specific ethnic groups and intermediate-risk profile patients. Hence, new biomarkers were developed to better stratify patients, such as the coronary artery calcium (CAC) score. This score is a preclinical marker of atherosclerosis, derived from cardiac computed tomography (CT) measurements [107]. The CAC score is an additional test recommended for refining the prediction of cardiovascular events. Nevertheless, CAC measurements remain invasive (radiation risk) and expensive; moreover, access to a cardiac CT system is needed. To tackle these issues, several research teams hypothesized that CAC could be estimated with retinal photograph-based DL. Son et al. presented a DL algorithm to discriminate patients with high CAC scores (threshold > 100 units) from patients with low CAC scores (CAC = 0) based on retinal fundus photographs [108]. They showed a moderate AUC of 0.823 with unilateral and 0.832 with bilateral fundus images. However, discrimination (CAC > 100 vs. CAC = 0) was better with the usual cardiovascular risk factors. Interestingly, a better performance was achieved with the combination of retinal fundus images and cardiovascular risk factors (age, presence of hypertension, gender), yielding an AUC of 0.886. In that respect, future algorithms based on retinal images should integrate clinical characteristics to improve their performance. Rim et al. [109] described a DL-based CAC score prediction from fundus photographs (RetiCAC). They hypothesized that the RetiCAC score could help in cardiovascular risk stratification and also in refining the stratification of groups of patients with borderline and intermediate PCE risk. The performance of RetiCAC was comparable to that of cardiac CT scans in predicting future CVD events. Furthermore, RetiCAC demonstrated interesting performance as an incremental feature to further stratify intermediate PCE groups. For example, in the borderline-risk group, RetiCAC could further stratify the risk of SCORE cardiovascular events (HR trend 1.62, 95% CI 1.04–2.54). Another strategy was presented by Chang et al. with prediction based on retinal fundus images of abnormal carotid artery intima-media thickness (CMIT) measured by means of ultrasonography [110]. In this study, CMIT was considered as the proxy marker for atherosclerosis. The authors aimed to predict, based on retinal fundus images, the DL-funduscopic atherosclerosis score (DL-FAS). The model was able to predict the ultrasonography-confirmed carotid artery atherosclerosis with an AUROC of 0.713. DL-FAS allowed the authors to refine the FRS calculation. In FRS subgroups of low or intermediate risk scores, the highest tercile of DL-FAS had significantly higher risk of cardiovascular mortality compared to the lowest tercile (HR, 95% CI 4.76, 1.05–21.63; 3.14, 1.04–9.47; respectively). Hence, DL-FAS could add to conventional risk stratification scores such as FRS for longitudinal outcomes of cardiovascular disease. These results supported the idea that imaging of the retinal vascular network and AI could enhance cardiovascular risk stratification based on historical risk score calculations (FRS, PCE and CAC score).
Prediction of Major Cardiovascular Events
Cheung et al. demonstrated a significant prediction rate of SIVA-DLS retinal arteriolar calibre for incident cardiovascular events (stroke, myocardial infarction, cardiovascular death) [37]. They showed that narrower central retinal arteriolar equivalent [HR per SD decrease, 1.13 (1.02–1.26)] was independently associated with incident CVD events, after adjusting for age, gender, ethnicity, fellow calibre, body mass index, MABP, glycated haemoglobin level, total cholesterol level and smoking at baseline. Poplin et al. trained a model to predict the onset of major adverse cardiovascular events (MACE) within 5 years [36]. Their model achieved an AUC of 0.70 (95% CI 0.65–0.74) from retinal fundus images. This prediction model alone was close to the AUC of 0.72 (95% CI 0.67–0.76) for the composite European SCORE risk calculator (SCORE risk) in this population [111]. Nevertheless, the association of the retinal fundus algorithm and SCORE risk calculation did not significantly improve the prediction of MACE.
Kawasaki et al. explored the explainability of this approach by developing a two-stage multitask DL network to output ten traditional CVD risk factors in the first stage and then estimate the 5-year MACE using the same UK Biobank dataset. By adopting this two-stage approach, the black box retinal image of the CVD event prediction model is easily interpreted by humans. Although the combination of the measured CVD risk factors and retinal images yielded the best performance [0.769 (95% CI 0.742–0.795)], this two-stage approach based on the retinal images alone has a relatively high accuracy with an AUC of 0.738 (95% CI 0.710–0.766), and it outperformed the SCORE/SCORE2 and FRS.
Nusinovici et al. developed a retinal fundus photography-based biological age (termed “RetiAGE”) algorithm [104]. This DL algorithm was trained on retinal fundus images to predict the likelihood of being > 65 years. The RetiAGE was compared with the patients’ actual chronological and phenotypic biomarkers (combination of chronological age and albumin, creatinine, glucose, C-reactive protein, lymphocyte percentage, red cell volume and distribution, alkaline phosphatase and white blood cell count) for the prediction of cardiovascular mortality and incidence of cardiovascular events. Independently of chronological age and phenotypic biomarkers, the authors reported a significant prediction rate for cardiovascular mortality (comparing the first and fourth quartile of RetiAGE) with an HR (adjusted for phenotypic and chronological age) of 2.42 (95% CI 1.69–3.48). Regarding the prediction of cardiovascular events, the HR (adjusted for phenotypic age) for the prediction rate was 1.39 (1.14–1.69). Moreover, an incremental increase in the predictive performance was found when adding the DL-predicted RetiAGE score to the risk models (model 1 = chronological age and model 3 = phenotypic age). However, this improvement remained minimal from 0.08 to 1.8% compared to model 1 and model 3, respectively. Retinal age prediction based on fundus photography was also investigated by Zhu et al., but they did not find any significant association with cardiovascular-related death [112]. Chang et al. presented a prediction model for cardiovascular mortality based on DL-FAS [110] and compared its performance with the FRS. Analysis of the relative integrated discrimination improvement (IDI) showed that the model using FRS risk levels plus DL-FAS had an IDI of 0.0007 (p = 0.008) and a relative IDI of 20.45% over the model using FRS risk levels alone for the prediction of cardiovascular mortality. This study found that prediction algorithm could add an incremental value to conventional risk stratification scores such as the Framingham Risk Score for major cardiovascular event (cardiovascular-related death). Diaz-Pinto et al. trained a multichannel variational autoencoder and a deep regressor model to estimate left ventricular mass and left ventricular end-diastolic volume from retinal fundus photographs [113]. Moreover, they aimed to predict incident myocardial infarction. To refine their algorithm, they added the patients’ demographic data to the model. This strategy could be a standard for future “oculomics” studies.
AI and Other Retinal Imaging Parameters
Currently, AI-based retinal biomarkers to predict cardiovascular risk profile are mainly represented by retinal fundus photographs. Nevertheless, other retinal imaging techniques could improve the performance of predicting models. There have been ongoing developments in OCT-A for a decade, which could facilitate detailed qualitative and quantitative descriptions of the retinal microvascular network [7]. OCT-A retinal vascular parameters were previously reported to have significant associations with cardiovascular profile [20], cardiovascular risk factors [22] and systemic vascular events [114, 115]; moreover, the technique has been widely adopted in international ophthalmology departments.
Recent advances in AI have improved the analysis of OCT-A images and the description of retinal vascular networks with these devices. For example, OCT-A-based studies presented their algorithms for automated disease detection (single and multiple retinal diseases: e.g., AMD, diabetic retinopathy, diabetic macular ischaemia and rare retinal diseases) [116,117,118,119].
To improve cardiovascular risk assessment, OCT-A-based research should focus on retinal vascular network analysis. Ma et al. presented their model, the Retinal OCTA SEgmentation dataset (ROSE), for automated segmentation of retinal vessels in OCT-A [120]. The challenge of distinguishing between arteries and veins on OCT-A has been solved recently [121], which could support research hypotheses on the association with systemic vascular disorders (more arterial or venous phenotype depending on which vascular component is affected). Furthermore, potential retinal biomarkers for cardiovascular assessment with AI such as automated foveal avascular zone measurement [122, 123], retinal vessel calibre and tortuosity measurements [124] have been introduced. These experimental algorithms could be used in future studies to investigate microvascular and macrovascular associations.
There is also growing interest in the use of ophthalmic adaptive optics (AO) for retinal vascular biomarkers [125]. A significant association of the retinal vascular structure (inner and outer diameter, parietal thickness) with hypertension has been found [126, 127]. Recently, Zhang et al. applied automatic segmentation of microaneurysms from AO with success [128]. Nevertheless, at present, AO remains a time-consuming, expensive and limited-access technology, which restricts its application to large population datasets.
There is no doubt that oculomics will benefit from retinal vascular parameters automatically extracted from OCT-A and AO in the near future.
Limitations of AI Approach
Methodological Issues
AI and medical imaging analysis are receiving increasing attention in all medical fields, including in ophthalmology and retinal vascular network imaging [129]. To develop and implement accurate clinical prediction models of cardiovascular risk profiles from retinal vascular network analysis, studies have to respect strict methodological rules. Replicability, reproducibility and generalizability should be the aim of each AI prediction model for cardiovascular risk assessment. Standard requirements (i.e., external validation datasets, opensource algorithms, rigorous independent labeling of retinal imaging and cardiovascular profiles) could help produce reliable results in the field. In that respect, the Equator network provided scientific guidelines (CONSORT-AI and SPIRIT-AI) to tackle inadequate reports in the field [130, 131]. Moreover, Van Smeden et al. presented 12 critical questions about AI-based prediction of CVD (i.e., are the data for prediction model development and testing representative for the targeted patient population and intended use? Is artificial intelligence needed to solve the targeted medical problem?) [132]. This checklist should be kept in mind when investigating AI-based retinal vascular biomarkers for the prediction of cardiovascular risk profile. Finally, it may be complicated to compare results from one study with another, since the authors have not always presented the performance of their algorithm with standardized methods and in a specific population dataset. This has to be improved to upgrade the quality of publications and the reliability of AI-based biomarkers. We could also raise ethical concerns. Fundus photographs could be presented as a potential future biometric safety measure. Hence, there may be ethical issues in the use of large open-source datasets.
Cost-Effectiveness and Availability
In assessing the use of AI-based retinal biomarkers to predict cardiovascular risk profiles, retinal imaging (fundus photographs and OCT-A) is compared with cardiovascular screening tools. On the one hand, retinal fundus photographs are less expensive, more widespread and less invasive than cardiac CT scans. On the other hand, risk evaluation scales based on clinical parameters such as the FRS and PCE continue to be inexpensive when measured during a routine consultation.
AI-based retinal biomarkers could be a cost-effective strategy in academic research and in daily clinical routine with general practitioner settings. It has a potential to expand the scope of cardiovascular disease assessment based on the retinal imaging and oculomics to general health screening programmes outside of the clinical practice (e.g., general health screening program for adults in Japan). However, the healthcare workflow, the proximity between the retinal imaging device and the cardiovascular unit and the availability of imaging algorithms, trained staff and backup data could increase costs. AI-based biomarkers promise to deliver more data, in a faster and cheaper way, but future studies are needed to focus on these economic aspects. To our knowledge, these points have not yet been investigated in the literature.
Incremental Value of AI-based Retinal Biomarkers
CVDs remain the leading cause of death globally: An estimated 17.9 million people died from CVDs in 2019 [133]. Hence, more sensitive and earlier biomarkers are needed to improve cardiovascular risk assessment and to individualize risk score calculation. Nevertheless, it has not yet been proven that AI-based retinal biomarkers can improve predictions over existing prediction models. Poplin et al. presented their results in predicting 5-year MACE. The AUC values from their analyses were 0.72, 0.70 and 0.72 for SCORE, an algorithm alone and SCORE+ an algorithm, respectively. Even if the results are very interesting, the clinical relevance of this difference could be questioned for future healthcare workflows.
Furthermore, in numerous studies the cut-off values used to train and validate the algorithm (i.e., CAC > 100 versus CAC = 0) are mostly anecdotal. These extreme cut-off values are far from patient outcomes and clinical workflow. Moreover, this method does not surpass prevailing prediction risk models (CAC, ankle-brachial index and family history) that consider intermediate risk profiles [34]. To confirm the incremental value of AI-based retinal biomarkers for cardiovascular risk prediction, future studies should be based on an interventional design. Hence, AI-based retinal biomarkers could motivate potential modification of patients’ therapy (statin or antiplatelet treatment). This strategy could be drawn from current protocols on referrals for medical retinal disease (134).
Conclusion
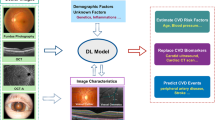
AI analysis based mainly on fundus photographs and OCT-A may strengthen the associations between retinal vascular network features and cardiovascular risk assessment. Hence, the associations between retinal microvasculature and systemic macrovasculature can be confirmed. A very high prediction rate and accuracy in performance were reached with numerous algorithms. Indeed, cardiovascular risk factors, cardiovascular risk stratification and major cardiovascular events could be predicted with rates up to 80%. Nevertheless, it remains unclear whether the AI approach outperforms traditional prediction models. Automated retinal vascular parameters could offer incremental value for specific targeted patient groups in the future (Fig. 1). Finally, these promising results from oculomics have been based on epidemiological population datasets, and they now need to confront real-world healthcare workflows.
Literature Research methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The literature search for this review was based on combining a set of keywords from the medical field (“ophthalmology”, “retina”, “fundus photographs”, “retinal vascular network”, “optical coherence tomography–angiography”, “adaptive optics”, “cardiovascular risk factors”, “cardiovascular events”, “cardiovascular prediction”, “cardiovascular risk stratification”, “oculomics”) with a set of keywords from the machine learning field (“artificial intelligence”, “deep learning” and convolutional neural network”). All terms from one set were independently combined with all terms from the other set. The main repository for the search were PubMed and Medline. English peer-reviewed articles until August 2022 deemed relevant (by inspection of the title and abstract) were reviewed (LA). The main inclusion criteria were the perceived quality of the research and the focus on AI, retinal vascular imaging, oculomics and cardiovascular status assessment. We acknowledge several limitations to this review methodology. First, we only selected perceived quality English peer-reviewed manuscript, which could have introduced a selection bias and a restriction of the research algorithm. Second, PRISMA checklist was not fully complete as we were not reporting a systematic review and meta-analyses.
References
Li H, Hsu W, Lee ML, Wong TY. Automatic grading of retinal vessel caliber. IEEE Trans Biomed Eng. 2005;52(7):1352–5.
Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology. 2008;115(11):1951–6.
Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BEK, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28(16):1984–92.
You QS, Chan JCH, Ng ALK, Choy BKN, Shih KC, Cheung JJC, et al. Macular vessel density measured with optical coherence tomography angiography and its associations in a large population-based study. Invest Ophthalmol Vis Sci. 2019;60(14):4830–7.
Lei J, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135(10):1092–8.
Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–32.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Sun Z, Yang D, Tang Z, Ng DS, Cheung CY. Optical coherence tomography angiography in diabetic retinopathy: an updated review. Eye. 2021;35(1):149–61.
Moghimi S, Hou H, Rao H, Weinreb RN. Optical coherence tomography angiography and glaucoma: a brief review. Asia Pac J Ophthalmol (Phila). 2019;8(2):115–25.
Forster RB, Garcia ES, Sluiman AJ, Grecian SM, McLachlan S, MacGillivray TJ, et al. Retinal venular tortuosity and fractal dimension predict incident retinopathy in adults with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetologia. 2021;64(5):1103–12.
Chiquet C, Gavard O, Arnould L, Mautuit T, Macgillivray TJ, Bron AM, et al. Retinal vessel phenotype in patients with primary open-angle glaucoma. Acta Ophthalmol. 2020;98(1):e88-93.
Monteiro-Henriques I, Rocha-Sousa A, Barbosa-Breda J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmol. 2022;100(1):e1-15.
Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121(21):2317–25.
Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, et al. The human microcirculation: regulation of flow and beyond. Circ Res. 2016;118(1):157–72.
Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57.
Wong TY, McIntosh R. Systemic associations of retinal microvascular signs: a review of recent population-based studies. Ophthalmic Physiol Opt. 2005;25(3):195–204.
Cheung CYL, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60(5):1094–103.
Wong TY, Klein R, Klein BEK, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46(1):59–80.
Arnould L, Binquet C, Guenancia C, Alassane S, Kawasaki R, Daien V, et al. Association between the retinal vascular network with Singapore «I»Vessel Assessment (SIVA) software, cardiovascular history and risk factors in the elderly: the Montrachet study, population-based study. PLoS ONE. 2018;13(4):e0194694.
Arnould L, Guenancia C, Azemar A, Alan G, Pitois S, Bichat F, et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59(10):4299–306.
Seidelmann SB, Claggett B, Bravo PE, Gupta A, Farhad H, Klein BE, et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the atherosclerosis risk in communities study. Circulation. 2016;134(18):1328–38.
Chua J, Chin CWL, Hong J, Chee ML, Le TT, Ting DSW, et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens. 2019;37(3):572.
Wagner SK, Fu DJ, Faes L, Liu X, Huemer J, Khalid H, et al. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol. 2020;9(2):6.
Wagner SK, Hughes F, Cortina-Borja M, Pontikos N, Struyven R, Liu X, et al. AlzEye: longitudinal record-level linkage of ophthalmic imaging and hospital admissions of 353 157 patients in London, UK. BMJ Open. 2022;12(3):e058552.
Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88.
Ting DSW, Cheung CYL, Lim G, Tan GSW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–23.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402–10.
Burlina PM, Joshi N, Pekala M, Pacheco KD, Freund DE, Bressler NM. Automated grading of age-related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. 2017;135(11):1170–6.
Thompson AC, Jammal AA, Medeiros FA. A review of deep learning for screening, diagnosis, and detection of glaucoma progression. Transl Vis Sci Technol. 2020;9(2):42.
Mursch-Edlmayr AS, Ng WS, Diniz-Filho A, Sousa DC, Arnould L, Schlenker MB, et al. Artificial intelligence algorithms to diagnose glaucoma and detect glaucoma progression: translation to clinical practice. Transl Vis Sci Technol. 2020;9(2):55.
D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53.
SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42(25):2439–54.
Pylypchuk R, Wells S, Kerr A, Poppe K, Riddell T, Harwood M, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391(10133):1897–907.
Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95.
Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJC, Asselbergs FW, Anderson TJ, et al. Race/ethnic differences in the associations of the framingham risk factors with carotid IMT and cardiovascular events. PLoS ONE. 2015;10(7):e0132321.
Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2(3):158.
Cheung CY, Xu D, Cheng CY, Sabanayagam C, Tham YC, Yu M, et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng. 2021;5(6):498–508.
Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920–30.
Noorbakhsh-Sabet N, Zand R, Zhang Y, Abedi V. Artificial intelligence transforms the future of healthcare. Am J Med. 2019;132(7):795–801.
Çetinkaya MB, Duran H. A detailed and comparative work for retinal vessel segmentation based on the most effective heuristic approaches. Biomed Tech (Berl). 2021;66(2):181–200.
Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29.
Decencière E, Zhang X, Cazuguel G, Lay B, Cochener B, Trone C, et al. Feedback on a publicly distributed image database: the messidor database. Image Anal Stereol. 2014;33(3):231–4.
Staal J, Abramoff MD, Niemeijer M, Viergever MA, van Ginneken B. Ridge-based vessel segmentation in color images of the retina. IEEE Trans Med Imaging. 2004;23(4):501–9.
Hoover AD, Kouznetsova V, Goldbaum M. Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans Med Imaging. 2000;19(3):203–10.
Decencière E, Cazuguel G, Zhang X, Thibault G, Klein JC, Meyer F, et al. TeleOphta: machine learning and image processing methods for teleophthalmology. IRBM. 2013;34(2):196–203.
Chalakkal RJ, Abdulla W. Automatic segmentation of retinal vasculature. In: 2017 IEEE international conference on acoustics, speech and signal processing (ICASSP). 2017. p. 886–90.
Khan SM, Liu X, Nath S, Korot E, Faes L, Wagner SK, et al. A global review of publicly available datasets for ophthalmological imaging: barriers to access, usability, and generalisability. Lancet Digit Health. 2021;3(1):e51-66.
Leishman R. The eye in general vascular disease: hypertension and arteriosclerosis. Br J Ophthalmol. 1957;41(11):641–701.
Parr JC. Hypertensive generalised narrowing of retinal arteries. Trans Ophthalmol Soc N Z. 1974;26:55–60.
Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol. 1974;77(4):478–83.
Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77(4):472–7.
Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–80.
Cheung CYL, Zheng Y, Hsu W, Lee ML, Lau QP, Mitchell P, et al. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 2011;118(5):812–8.
Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011;54(9):2409–16.
Sasongko MB, Wang JJ, Donaghue KC, Cheung N, Benitez-Aguirre P, Jenkins A, et al. Alterations in retinal microvascular geometry in young Type 1 Diabetes. Diabetes Care. 2010;33(6):1331–6.
Liew G, Mitchell P, Rochtchina E, Wong TY, Hsu W, Lee ML, et al. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J. 2011;32(4):422–9.
Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29(7):1380–91.
Patton N, Aslam TM, MacGillivray T, Deary IJ, Dhillon B, Eikelboom RH, et al. Retinal image analysis: concepts, applications and potential. Prog Retin Eye Res. 2006;25(1):99–127.
Kawasaki R, Che Azemin MZ, Kumar DK, Tan AG, Liew G, Wong TY, et al. Fractal dimension of the retinal vasculature and risk of stroke: a nested case-control study. Neurology. 2011;76(20):1766–7.
Wong TY, Knudtson MD, Klein R, Klein BEK, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–90.
Ding J, Wai KL, McGeechan K, Lkram MK, Kawasaki R, Xie J, et al. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014;32(2):207–15.
Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, et al. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27(12):2386–93.
Ponto K, Werner D, Wiedemer L, Laubert-Reh D, Schuster A, Nickels S, et al. Retinal vessel metrics: normative data and their use in systemic hypertension results from the Gutenberg Health Study. J Hypertens. 2017;35(8):1635–45.
Sng CCA, Wong WL, Cheung CY, Lee J, Tai ES, Wong TY. Retinal vascular fractal and blood pressure in a multiethnic population. J Hypertens. 2013;31(10):2036–42.
Jeganathan VSE, Sabanayagam C, Tai ES, Lee J, Sun C, Kawasaki R, et al. Effect of blood pressure on the retinal vasculature in a multi-ethnic Asian population. Hypertens Res. 2009;32(11):975–82.
Wong TY, Klein R, Nieto FJ, Klein BEK, Sharrett AR, Meuer SM, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–40.
Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–81.
Liew G, Gopinath B, White AJ, Burlutsky G, Yin Wong T, Mitchell P. Retinal vasculature fractal and stroke mortality. Stroke. 2021;52(4):1276–82.
Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, et al. Retinal fractals and acute lacunar stroke. Ann Neurol. 2010;68(1):107–11.
McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Sharrett AR, et al. Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol. 2008;102(1):58–63.
Ikram MK, de Jong FJ, Vingerling JR, Witteman JCM, Hofman A, Breteler MMB, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? the rotterdam study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–34.
Wong TY, Wang JJ, Rochtchina E, Klein R, Mitchell P. Does refractive error influence the association of blood pressure and retinal vessel diameters? the blue mountains eye study. Am J Ophthalmol. 2004;137(6):1050–5.
Xing C, Klein BEK, Klein R, Jun G, Lee KE, Iyengar SK. Genome-wide linkage study of retinal vessel diameters in the beaver dam eye study. Hypertension. 2006;47(4):797–802.
Sun C, Liew G, Wang JJ, Mitchell P, Saw SM, Aung T, et al. Retinal vascular caliber, blood pressure, and cardiovascular risk factors in an Asian population: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49(5):1784–90.
McGrory S, Taylor AM, Pellegrini E, Ballerini L, Kirin M, Doubal FN, et al. Towards Standardization of Quantitative Retinal Vascular Parameters: Comparison of SIVA and VAMPIRE Measurements in the Lothian Birth Cohort 1936. Transl. Vis. Sci. Technol. 2018;7(2).
Downie E, Tokarev J, Divani A, Koozekanani DD. Comparison of two free retinal vascular measurement software packages: IVAN and VAMPIRE. Invest Ophthalmol Vis Sci. 2015;56(7):3320–3320.
Mautuit T, Semecas R, Hogg S, Daien V, Gavard O, Chateau N, et al. Comparing measurements of vascular diameter using adaptative optics imaging and conventional fundus imaging. Diagnostics (Basel). 2022;12(3):705.
Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–48.
Krestanova A, Kubicek J, Penhaker M. Recent techniques and trends for retinal blood vessel extraction and tortuosity evaluation: a comprehensive review. IEEE Access. 2020;8:197787–816.
Shi D, Lin Z, Wang W, Tan Z, Shang X, Zhang X, et al. A deep learning system for fully automated retinal vessel measurement in high throughput image analysis. Front Cardiovasc Med. 2022;9:823436.
Guo S, Wang K, Kang H, Zhang Y, Gao Y, Li T. BTS-DSN: deeply supervised neural network with short connections for retinal vessel segmentation. Int J Med Inform. 2019;126:105–13.
Kim G, Kim J, Choi WJ, Kim C, Lee S. Integrated deep learning framework for accelerated optical coherence tomography angiography. Sci Rep. 2022;12(1):1289.
Hasan MK, Alam MA, Elahi MTE, Roy S, Martí R. DRNet: Segmentation and localization of optic disc and Fovea from diabetic retinopathy image. Artif Intell Med janv. 2021;111:102001.
Tang S, Qi Z, Granley J, Beyeler M. U-Net with Hierarchical Bottleneck Attention for Landmark Detection in Fundus Images of the Degenerated Retina. 2021. 62-71. Available on: http://arxiv.org/abs/2107.04721
Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. 2015. http://arxiv.org/abs/1505.04597
Fu H, Xu Y, Lin S, Kee Wong DW, Liu J. DeepVessel: Retinal Vessel Segmentation via Deep Learning and Conditional Random Field. In: Ourselin S, Joskowicz L, Sabuncu MR, Unal G, Wells W, éditeurs. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2016. Cham: Springer International Publishing; 2016. p. 132–9.
Mou L, Chen L, Cheng J, Gu Z, Zhao Y, Liu J. Dense dilated network with probability regularized walk for vessel detection. IEEE Trans Med Imaging. 2020;39(5):1392–403.
Yue K, Zou B, Chen Z, Liu Q. Retinal vessel segmentation using dense U-net with multiscale inputs. J Med Imaging (Bellingham). 2019;6(3):034004.
Zihe Huang, Ying Fang, He Huang et al. Automatic Retinal Vessel764
Segmentation Based on an Improved U-Net Approach. https://www.hindawi.com/journals/sp/2021/5520407/
Du XF, Wang JS, Sun WZ. UNet retinal blood vessel segmentation algorithm based on improved pyramid pooling method and attention mechanism. Phys Med Biol. 2021;66(17).
Liu C, Penghui G, Xiao Z. Multiscale U-Net with Spatial770 Positional Attention for Retinal Vessel Segmentation. J Healthc Eng. 2022;2022:5188362.
Yan Z, Yang X, Cheng KT. A three-stage deep learning model for accurate retinal vessel segmentation. IEEE J Biomed Health Inform. 2019;23(4):1427–36.
Cao H, Wang Y, Chen J, Jiang D, Zhang X, Tian Q, et al. Swin-Unet: Unet-like Pure Transformer for Medical Image Segmentation. 2021. http://arxiv.org/abs/2105.05537
Danny C, Wenzhong Y, Liejun W, Sixiang T, Jiangzhaung L, Wenxiu B. PCAT-UNet: UNet-like network fused convolution and transformer for retinal vessel segmentation. PLoS ONE. 2022;17(1):e0262689.
Welikala RA, Fraz MM, Habib MM, Daniel-Tong S, Yates M, Foster PJ, et al. Automated quantification of retinal vessel morphometry in the UK biobank cohort. In: 2017 Seventh International Conference on Image Processing Theory, Tools and Applications (IPTA). 2017. p. 1–6.
Welikala RA, Foster PJ, Whincup PH, Rudnicka AR, Owen CG, Strachan DP, et al. Automated arteriole and venule classification using deep learning for retinal images from the UK Biobank cohort. Comput Biol Med. 2017;90:23–32.
Tapp RJ, Owen CG, Barman SA, Welikala RA, Foster PJ, Whincup PH, et al. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness: United Kingdom Biobank. Hypertension. 2019;74(6):1383–90.
Rudnicka AR, Welikala R, Barman S, Foster PJ, Luben R, Hayat S, et al. Artificial intelligence-enabled retinal vasculometry for prediction of circulatory mortality, myocardial infarction and stroke. Br J Ophthalmol. 2022;bjophthalmol-2022–321842.
Dhodapkar RM, Li E, Nwanyanwu K, Adelman R, Krishnaswamy S, Wang JC. Deep learning for quality assessment of optical coherence tomography angiography images. Sci Rep. 2022;12(1):13775.
Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, et al. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40(25):2058–73.
Kim YD, Noh KJ, Byun SJ, Lee S, Kim T, Sunwoo L, et al. Effects of hypertension, diabetes, and smoking on age and sex prediction from retinal fundus images. Sci Rep. 2020;10(1):4623.
Arnould L, Guenancia C, Bourredjem A, Binquet C, Gabrielle PH, Eid P, et al. Prediction of cardiovascular parameters with supervised machine learning from Singapore «I» vessel assessment and OCT-angiography: a pilot study. Transl Vis Sci Technol. 2021;10(13):20.
Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741-752.
Nusinovici S, Rim TH, Yu M, Lee G, Tham YC, Cheung N, et al. Retinal photograph-based deep learning predicts biological age, and stratifies morbidity and mortality risk. Age Ageing. 2022;51(4):afac065.
Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83(1):356–62.
Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45.
Son J, Shin JY, Chun EJ, Jung KH, Park KH, Park SJ. Predicting high coronary artery calcium score from retinal fundus images with deep learning algorithms. Trans Vis Sci Tech. 2020;9(2):28–28.
Rim TH, Lee CJ, Tham YC, Cheung N, Yu M, Lee G, et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit Health. 2021;3(5):e306–16.
Chang J, Ko A, Park SM, Choi S, Kim K, Kim SM, et al. Association of cardiovascular mortality and deep learning-funduscopic atherosclerosis score derived from retinal fundus images. Am J Ophthalmol. 2020;217:121–30.
Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003.
Zhu Z, Shi D, Guankai P, Tan Z, Shang X, Hu W, et al. Retinal age gap as a predictive biomarker for mortality risk. Br J Ophthalmol. 2022;bjophthalmol-2021–319807.
Diaz-Pinto A, Ravikumar N, Attar R, Suinesiaputra A, Zhao Y, Levelt E, et al. Predicting myocardial infarction through retinal scans and minimal personal information. Nat Mach Intell. 2022;4(1):55–61.
Wang J, Jiang J, Zhang Y, Qian YW, Zhang JF, Wang ZL. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed Opt Express. 2019;10(4):1532–44.
Alan G, Guenancia C, Arnould L, Azemar A, Pitois S, Maza M, et al. Retinal vascular density as a novel biomarker of acute renal injury after acute coronary syndrome. Sci Rep. 2019;9(1):1–9.
Treder M, Lauermann JL, Eter N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefes Arch Clin Exp Ophthalmol. 2018;256(2):259–65.
Heisler M, Karst S, Lo J, Mammo Z, Yu T, Warner S, et al. Ensemble deep learning for diabetic retinopathy detection using optical coherence tomography angiography. Transl Vis Sci Technol. 2020;9(2):20.
Yoo TK, Choi JY, Kim HK. Feasibility study to improve deep learning in OCT diagnosis of rare retinal diseases with few-shot classification. Med Biol Eng Comput. 2021;59(2):401–15.
Yang D, Sun Z, Shi J, Ran A, Tang F, Tang Z, et al. A multitask deep-learning system for assessment of diabetic macular ischemia on optical coherence tomography angiography images. Retina. 2022;42(1):184–94.
Ma Y, Hao H, Xie J, Fu H, Zhang J, Yang J, et al. ROSE: a retinal OCT-angiography vessel segmentation dataset and new model. IEEE Trans Med Imaging. 2021;40(3):928–39.
Alam M, Alam M, Le D, Le D, Son T, Lim JI, et al. AV-Net: deep learning for fully automated artery-vein classification in optical coherence tomography angiography. Biomed Opt Express. 2020;11(9):5249–57.
Lin A, Fang D, Li C, Cheung CY, Chen H. Improved automated foveal avascular zone measurement in cirrus optical coherence tomography angiography using the level sets macro. Transl Vis Sci Technol. 2020;9(12):20.
Mirshahi R, Anvari P, Riazi-Esfahani H, Sardarinia M, Naseripour M, Falavarjani KG. Foveal avascular zone segmentation in optical coherence tomography angiography images using a deep learning approach. Sci Rep. 2021;11(1):1031.
Gao M, Guo Y, Hormel TT, Tsuboi K, Pacheco G, Poole D, et al. A deep learning network for classifying arteries and veins in montaged widefield OCT angiograms. Ophthalmol Sci. 2022;2(2):100149.
Bakker E, Dikland FA, van Bakel R, Andrade DJD, Sánchez BL, Klein S, et al. Adaptive optics ophthalmoscopy: a systematic review of vascular biomarkers. Surv Ophthalmol. 2022;67(2):369–87.
Rosenbaum D, Mattina A, Koch E, Rossant F, Gallo A, Kachenoura N, et al. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J Hypertens. 2016;34(6):1115–22.
Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud P, Girerd X, et al. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens. 2014;32(4):890–8.
Zhang Q, Sampani K, Xu M, Cai S, Deng Y, Li H, et al. AOSLO-net: a deep learning-based method for automatic segmentation of retinal microaneurysms from adaptive optics scanning laser ophthalmoscopy images. Transl Vis Sci Technol. 2022;11(8):7.
Boudry C, Al Hajj H, Arnould L, Mouriaux F. Analysis of international publication trends in artificial intelligence in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2022;260(5):1779–88.
Liu X, Rivera SC, Moher D, Calvert MJ, Denniston AK. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension. BMJ. 2020;370:m3164.
Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351–63.
van Smeden M, Heinze G, Van Calster B, Asselbergs FW, Vardas PE, Bruining N, et al. Critical appraisal of artificial intelligence-based prediction models for cardiovascular disease. Eur Heart J. 2022;ehac238.
Cardiovascular diseases (CVDs) [Internet]. [cité 22 oct 2019]. Disponible sur: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
Han JED, Liu X, Bunce C, Douiri A, Vale L, Blandford A, et al. Teleophthalmology-enabled and artificial intelligence-ready referral pathway for community optometry referrals of retinal disease (HERMES): a Cluster Randomised Superiority Trial with a linked Diagnostic Accuracy Study-HERMES study report 1-study protocol. BMJ Open. 2022;12(2):e055845.
Acknowledgements
Funding
This work was supported by a grant from Fondation de France; Council for Science, Technology and Innovation, Cross-ministerial Strategic Innovation Promotion Program: "Innovative AI Hospital System" (Funding Agency: National Institute of Biomedical Innovation, Health and Nutrition). No funding was received for the publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing and/or Editorial Assistance
English editing by Isabella Athanassiou, BA Mphil (copyediting/proofreading) Isabella3@t-online.de.
Author Contributions
Louis Arnould: collected the data, wrote the paper; Fabrice Meriaudeau: designed the analysis; Charles Guenancia: edited and revised the manuscript; Clément Germanese: wrote the paper; Cécile Delcourt: edited and revised the manuscript; Ryo Kawasaki: edited and revised the manuscript; Carol Cheung: contributed data and revised the manuscript; Catherine Creuzot-Garcher: edited and revised the manuscript; Andrzej Grzybowski: designed the project.
Disclosures
Louis Arnould has nothing to disclose, Fabrice Meriaudeau has nothing to disclose, Charles Guenancia has nothing to disclose, and Clément Germanese has nothing to disclose. Cécile Delcourt: Consultant for Allergan, Chauvin-Bausch+Lomb, Théa Pharma and Novartis, grants from Théa Pharma, speaker fees from Apellis. R Kawasaki: Topcon (Endowed Department). Carol Cheung has nothing to disclose; Catherine Creuzot-Garcher has nothing to disclose; A Grzybowski: grants—Alcon, Bausch&Lomb, Zeiss, Teleon, J&J, CooperVision, Hoya. Lectures: Thea, Polpharma, Viatris. Member of Advisory Boards: Nevakar, GoCheckKids and Thea.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Arnould, L., Meriaudeau, F., Guenancia, C. et al. Using Artificial Intelligence to Analyse the Retinal Vascular Network: The Future of Cardiovascular Risk Assessment Based on Oculomics? A Narrative Review. Ophthalmol Ther 12, 657–674 (2023). https://doi.org/10.1007/s40123-022-00641-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00641-5