Abstract
Purpose
Our purpose was to use deep learning for the automated detection of age-related macular degeneration (AMD) in spectral domain optical coherence tomography (SD-OCT).
Methods
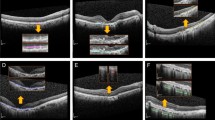
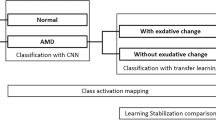
A total of 1112 cross-section SD-OCT images of patients with exudative AMD and a healthy control group were used for this study. In the first step, an open-source multi-layer deep convolutional neural network (DCNN), which was pretrained with 1.2 million images from ImageNet, was trained and validated with 1012 cross-section SD-OCT scans (AMD: 701; healthy: 311). During this procedure training accuracy, validation accuracy and cross-entropy were computed. The open-source deep learning framework TensorFlow™ (Google Inc., Mountain View, CA, USA) was used to accelerate the deep learning process. In the last step, a created DCNN classifier, using the information of the above mentioned deep learning process, was tested in detecting 100 untrained cross-section SD-OCT images (AMD: 50; healthy: 50). Therefore, an AMD testing score was computed: 0.98 or higher was presumed for AMD.
Results
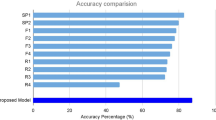
After an iteration of 500 training steps, the training accuracy and validation accuracies were 100%, and the cross-entropy was 0.005. The average AMD scores were 0.997 ± 0.003 in the AMD testing group and 0.9203 ± 0.085 in the healthy comparison group. The difference between the two groups was highly significant (p < 0.001).
Conclusions
With a deep learning-based approach using TensorFlow™, it is possible to detect AMD in SD-OCT with high sensitivity and specificity. With more image data, an expansion of this classifier for other macular diseases or further details in AMD is possible, suggesting an application for this model as a support in clinical decisions. Another possible future application would involve the individual prediction of the progress and success of therapy for different diseases by automatically detecting hidden image information.





Similar content being viewed by others
References
Chou CF, Cotch MF, Vitale S, Zhang X, Klein R, Friedman DS, Klein BE, Saaddine JB (2013) Age-related eye diseases and visual impairment among U.S. adults. Am J Prev Med 45:29–35
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR, Beckman Initiative for Macular Research Classification C (2013) Clinical classification of age-related macular degeneration. Ophthalmology 120:844–851
Huang D, Swanson E, Lin C, Schuman J, Stinson W, Chang W, Hee M, Flotte T, Gregory K, Puliafito C, Fujimoto J (1991) Optical coherence tomography. Science 254:1178–1181
Regatieri C, Branchini L, Duker J (2011) The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 42 Suppl:S56-66
Lim E-H, Han J, Kim C, Cho S, Lee T (2013) Characteristic findings of optical coherence tomography in retinal Angiomatous proliferation. Korean J Ophthalmol 27:351–360
Cicinelli MV, Rabiolo A, Sacconi R, Carnevali A, Querques L, Bandello F, Querques G (2017) Optical coherence tomography angiography in dry age-related macular degeneration. Surv Ophthalmol. https://doi.org/10.1016/j.survophthal.2017.06.005
Rampasek L, Goldenberg A (2016) TensorFlow: Biology's gateway to deep learning? Cell systems 2(1):12–14. https://doi.org/10.1016/j.cels.2016.01.009
van Ginneken B (2017) Fifty years of computer analysis in chest imaging: rule-based, machine learning, deep learning. Radiol Phys Technol 10:23–32
Bogunovic H, Waldstein S, Schlegl T, Langs G, Sadeghipour A, Liu X, Gerendas B, Osborne A, Schmidt-Erfurth U (2017) Prediction of anti-VEGF treatment requirements in neovascular AMD using a machine learning approach. Invest Ophthalmol Vis Sci 58:3240–4248
ElTanboly A, Ismail M, Shalaby A, Switala A, El-Baz A, Schaal S, Gimel'farb G, El-Azab M (2017) A computer-aided diagnostic system for detecting diabetic retinopathy in optical coherence tomography images. Med Phys 44:914–923
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR (2016) Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316:2402–2410
Abramoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, Niemeijer M (2016) Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 57:5200–5206
Burlina P, Pacheco KD, Joshi N, Freund DE, Bressler NM (2017) Comparing humans and deep learning performance for grading AMD: a study in using universal deep features and transfer learning for automated AMD analysis. Comput Biol Med 82:80–86
Wang Y, Zhang Y, Yao Z, Zhao R, Zhou F (2017) Machine learning based detection of age-related macular degeneration (AMD) and diabetic macular edema (DME) from optical coherence tomography (OCT) images. Biomed Opt Express 7:4928–4940
Gao S, Patel R, Jain N, Zhang M, Weleber R, Huang D, Pennesi M, Jia Y (2016) Choriocapillaris evaluation in choroideremia using optical coherence tomography angiography. Biomed Opt Express 8:48–56
Gerendas BS, Bogunovic H, Sadeghipour A, Schlegl T, Langs G, Waldstein SM, Schmidt-Erfurth U (2017) Computational image analysis for prognosis determination in DME. Vis Res. https://doi.org/10.1016/j.visres.2017.03.008
Venhuizen F, van Ginneken B, van Asten F, van Grinsven M, Fauser S, Hoyng C, Theelen T, Sánchez C (2017) Automated staging of age-related macular degeneration using optical coherence tomography. Invest Ophthalmol Vis Sci 58:2318–2328
Miri MS, Abramoff MD, Kwon YH, Sonka M, Garvin MK (2017) A machine-learning graph-based approach for 3D segmentation of Bruch's membrane opening from glaucomatous SD-OCT volumes. Med Image Anal 39:206–217
Alsaih K, Lemaitre G, Rastgoo M, Massich J, Sidibe D, Meriaudeau F (2017) Machine learning techniques for diabetic macular edema (DME) classification on SD-OCT images. Biomed Eng Online 16:68
Waldstein SM, Montuoro A, Podkowinski D, Philip AM, Gerendas BS, Bogunovic H, Schmidt-Erfurth U (2017) Evaluating the impact of vitreomacular adhesion on anti-VEGF therapy for retinal vein occlusion using machine learning. Sci Rep 7:2928
Vogl W, Waldstein S, Gerendas B, Schmidt-Erfurth U, Langs G (2017) Predicting macular edema recurrence from Spatio-Temporal signatures in optical coherence tomography images. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2017.2700213
Montuoro A, Waldstein S, Gerendas B, Schmidt-Erfurth U, Bogunović H (2017) Joint retinal layer and fluid segmentation in OCT scans of eyes with severe macular edema using unsupervised representation and auto-context. Biomed Opt Express 8:1874–1888
Kim S, Cho K, Oh S (2017) Development of machine learning models for diagnosis of glaucoma. PLoS One 12:e0177726
Murugeswari S, Sukanesh R (2017) Investigations of severity level measurements for diabetic macular oedema using machine learning algorithms. Ir J Med Sci. https://doi.org/10.1007/s11845-017-1598-8
Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, Corrado G, Davis A, Dean J, Devin M, Ghemawat S, Goodfellow I, Harp A, Irving G, Isard M, Jia Y, Jozefowicz R, Kaiser L, Kudlur M, Levenberg J, Mane′ D, Monga R, Moore S, Murray D, Olah C, Schuster M, Shlens J, Steiner B, Sutskever I, Talwar K, Tucker P, Vanhoucke V, Vasudevan V, Vie′ gas F, Vinyals O, Warden P, Wattenberg M, Wicke M, Yu Y, Zheng X (2015) TensorFlow: Large-scale machine learning on heterogeneous distributed systems. TensorFlow. https://static.googleusercontent.com/media/research.google.com/en//pubs/archive/45166.pdf. Accessed 4 June 2017
Szegedy C, Vanhoucke V, Ioffe S, Shlens J (2016) Rethinking the inception architecture for computer vision. IEEE Conf Comput Vis Pattern Recognit (CVPR) 2016:2818–2826
Deng J, Dong W, Socher R, Li L-J, Li K, Fei-Fei L (2009) ImageNet- a large-scale hierarchical image database. CVPR 2009 - IEEE Conf Comput Vis Pattern Recognit 2009:248–255
TensorFlow (2017) http://www.tensorflow.org/tutorials/image_recognition. TensorFlow. Accessed 26 June 2017
Google Developers (2017) https://codelabs.developers.google.com/codelabs/tensorflow-for-poets/#0. Google Developers. Accessed 4 July 2017
Angermueller C, Parnamaa T, Parts L, Stegle O (2016) Deep learning for computational biology. Mol Syst Biol 12:878
Lakhani P, Sundaram B (2017) Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using Convolutional neural networks. Radiology 284:574–582
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations
with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Financial disclosure
M. Treder: Allergan, Novartis; J.L. Lauermann: Bayer, Novartis; and N. Eter: Allergan, Alimera, Bausch and Lomb, Bayer, Heidelberg Engineering, Novartis, Roche.
Ethical approval
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Treder, M., Lauermann, J.L. & Eter, N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefes Arch Clin Exp Ophthalmol 256, 259–265 (2018). https://doi.org/10.1007/s00417-017-3850-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3850-3