Abstract
Introduction
Recent studies indicate that short-term monocular deprivation increases the deprived eye’s contribution to binocular fusion in both adults with normal vision and amblyopia. In this study, we investigated whether the changes in visual plasticity depended on the duration of deprivation in normal and amblyopic adults.
Methods
Twelve anisometropia amblyopic observers (aged 24.8 ± 2.3 years) and 12 age-matched normal observers (aged 23.9 ± 1.2 years) participated in the study. The non-dominant eye of normal observers or amblyopic eye of amblyopic observers was deprived for 30, 120, and 300 min in a randomized order. Their eye balance was measured with a phase combination task, which is a psychophysical test, before and after the deprivation. This design enabled us to measure changes induced in binocular balance as an index visual plasticity due to monocular deprivations.
Results
By comparing the ocular dominance changes as a result of monocular deprivation with different deprivation durations, we found evidence that the ocular dominance changes are slightly larger after longer deprivations in both normal and amblyopic observers, albeit with a statistical significance. The changes from 120-min were significantly greater than those from 30-min deprivation in both groups. The magnitude of changes in sensory eye balance was significantly larger in normal observers than that in the amblyopic observers; however, the longevity of changes in visual plasticity was found to be more long-lasting in amblyopic observers than the normal counterparts.
Conclusions
The duration of deprivation matters in both normal and amblyopic observers. Ocular dominance imbalance that is typically observed in amblyopia can be more ameliorated with a longer duration of deprivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent studies indicate that the deprivation of the poor eye benefits binocular visual functions, such as stereopsis and balance, in adults with amblyopia. |
Whether the binocular benefit can be greater by increasing the duration of deprivation remains elusive. |
Our results show that the sensory change after deprivation is larger as a function of the deprivation duration in both normal and amblyopic observers. |
Our findings could facilitate both clinicians and researchers to better design patching protocols and provide a more effective binocular treatment for amblyopia. |
Introduction
Amblyopia is a developmental disorder that is associated with abnormal visual experience early in life. It is a leading cause of unilateral visual deficit in children [1,2,3], affecting about 3% of the population [4]. Visual deprivation of pattern information or misalignment between the eyes during the critical period can cause amblyopia, which is optically uncorrectable and exhibits no pathology in the eye itself [5]. Besides monocular visual deficits, the amblyopic visual system has weakened excitatory connections that are important for stereopsis and binocular summation [6, 7]. On the other hand, the inhibitory connections seem to be anomalously strengthened in amblyopia; this is illustrated with the abnormal suppression between the two eyes. For the amblyopic observer to experience a reduced interocular suppression and thereby achieve binocular fusion, the contrast of the image shown to the fellow eye has to be reduced. In other words, a more severe suppression from the fellow eye to the amblyopic eye shifts the binocular balance in favor of the fellow eye [8, 9], thereby causing the amblyopic observers to be functionally monocular even if binocularity is still intact in amblyopia [9,10,11]. This form of perceptual suppression compromises binocular vision, impairing fusion between the eyes [8, 12,13,14]. An intact binocular vision is important for locating an object, perceiving depth, and guiding fine motor movements, all of which pervade everyday visual experiences. Moreover, amblyopic individuals exhibit abnormal eye movements [15], hand–eye coordination [16], reading speed [17, 18], and a low physical self-perception [19, 20]. In the last quarter of a millennium, therapy for amblyopia has targeted on improving the visual acuity of the amblyopic eye [21, 22]. However, even if the amblyopic eye achieves a full recovery in visual acuity, binocular visual functions might still not be significantly improved. For this reason, modern therapies, such as dichoptic gaming and movie treatments and perceptual learning, have been developed with the goal of restoring binocular functions [13, 23, 24].
Short-term monocular deprivation can strengthen the deprived eye’s contribution to binocular vision [25, 26]. This neural phenomenon involves homeostatic mechanisms in the visual cortex [27, 28]. Although the change in sensory eye balance from the deprivation is short-lived in normal adults, a study shows that its life span is noticeably longer in amblyopic observers [29]. The clinical relevance of this finding is that, if the amblyopic eye is deprived, the subsequent strengthening of the amblyopic eye’s contribution to binocular vision could represent an important new type of supplement to binocular therapy possibly by alleviating the abnormal interocular suppression. Moreover, unlike the current patching of the fellow eye, this new treatment protocol would not be met with such resistance in the pediatric amblyopic population since the deprived eye would be the amblyopic eye, rather than the fellow eye [30]. It is also important to bear in mind that the standard treatment of patching the fellow eye has a slightly different aim than the inverse occlusion that is presented in this study. The aim of the standard patching therapy is to improve the visual acuity of the amblyopic eye by increasing its use [31]. On the other hand, the goal of the inverse deprivation, which involves patching the amblyopic eye and has been recently recognized as a new form of therapy, is to improve binocular functions, such as fusion and balance. Despite their differences, two recent laboratory trials have demonstrated the effectiveness of amblyopic eye patching in adult patients over a 2-month period [32, 33]. Both show long-term binocular and monocular visual improvements that last up to 1 year. Suppression can impact the process of binocular combination, during which the inputs to each eye become fused. Therefore, abnormal interocular suppression in amblyopia might be mitigated to facilitate a more balanced binocular fusion by depriving the fellow eye [34].
One fundamental consideration in the development of a new patching treatment is the duration of patching that should be used each day. Although normal and amblyopic observers both display a change in their binocular balance after 1–2 h of monocular deprivation that lasts between 30 and 60 min, it is not known how the magnitude of this binocular imbalance changes with the duration of monocular deprivation. In normal observers, there is a surprisingly small dependence of the change in either the magnitude or the longevity of the binocular effect with duration of deprivation between 15 min and 5 h [35, 36]; however, this study has a small sample size and unpaired subjects across patching durations. For any clinical application of this approach to amblyopia, one needs to know how the rebalancing of binocular vision (amblyopic eye being patched) depends on the duration of patching, as we cannot assume it would the same as for normal vision. In fact, studies show that neural plasticity in the adult amblyopic visual system can be more potent than in the normal visual system [29, 37]. We set out to answer this question using an approach that has recently been shown to be optimal for this type of ocular dominance assessment [38].
In this study, we investigated whether the changes in visual plasticity would depend on the duration of deprivation in 12 amblyopic observers and 12 normal controls. We examined three durations of patching, 30, 120, and 300 min for each observer, who underwent about 15–16 h of testing in total. The aim of the study was to investigate whether the neuroplastic changes after inverse deprivation varied in their magnitudes as a function of the deprivation duration and to examine whether these changes significantly differed between normal and amblyopic observers. As a result of the recent finding that there is a weak relationship between the duration of inverse patching and its subsequent change in sensory eye balance in normal observers, we hypothesized that the duration of deprivation would not increasingly shift the sensory eye balance in both normal and amblyopic observers. Also, in light of the study that shows that changes in sensory eye balance are larger in amblyopia [29], we predicted that both the magnitude and longevity of the changes would be larger in amblyopia as a function of patching durations compared to those of normal controls [29].
Methods
Participants
In this study, 12 normal observers (aged 23.9 ± 1.2 years, 10 females) and 12 anisometropia amblyopic observers (aged 24.8 ± 2.3 years, 7 females) were recruited from the Affiliated Eye Hospital of Wenzhou Medical University. The clinical details of the patients are provided in Table 1. Amblyopia was defined on the basis of the Preferred Practice Patterns of the American Academy of Ophthalmology [30]. For the subjects to be categorized as having amblyopia, their interocular difference of best-corrected visual acuity between the amblyopic eye and the fellow eye had to be 0.20 logMAR or more. The ophthalmologists at the hospital performed standard visual assessments to properly diagnose amblyopia and confirm that no other ocular pathology existed that could explain their reduced vision. The amblyopic observers had no obvious ocular disease or structural abnormalities, and demonstrated a normal fixation in the center of the visual field. They were asked to wear spectacles so that the refractive errors could be corrected throughout testing. The normal observers showed a normal visual acuity (0.00 logMAR or better), stereoacuity (≤ 60 arcsecs), and no history of ocular disease or pertinent surgery. All subjects (normal controls and subjects with amblyopia) were naïve to the purpose of the experiment and provided written informed consent. This study is in line with the Declaration of Helsinki and was approved by the Institutional Review Boards at McGill University and Wenzhou Medical University. Each subject underwent about 15–16 h of testing. The dominant eye was determined with a pinhole test [39], the visual acuity was assessed using the ETDRS visual acuity chart, and the stereoacuity was measured using a Random Dot stereo test (RDS).
Procedure
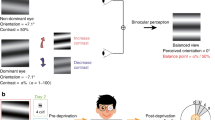
The procedure of the experimental design is outlined in Fig. 1. We first obtained each subject’s balance point with the binocular phase combination task (Fig. 2). Before patching, the subjects were asked to perform three blocks of baseline experiment, each of which lasted for about three minutes. The binocular balance of all subjects was measured before patching. After completing the baseline test, participants were asked to place a translucent patch on their amblyopic eye (or non-dominant eye of normal observers) for either 30, 120, or 300 min (Fig. 1). These three durations were chosen on the basis of the design of the previous study that reports a weak relationship between patching duration and its effect on changes in sensory eye dominance after patching [35]. We randomized the order of the three conditions, each of which was completed on separate days. During patching, the subjects performed ordinary office tasks such as web browsing through their computer or phone; their activities were not standardized but the observers were asked to stay within the lab throughout the experiment. After patching, the subjects performed the same visual test at 0, 3, 6, 12, 26, and 60 min (Fig. 1).
Experimental design. Three sessions of baseline measurement were conducted. Then the non-dominant eye (NDE) of normal controls and the amblyopic eye (AE) of subjects with amblyopia were patched for either 30, 120, or 300 min (completed in a randomized order on separate days). Next, they performed post-patching test at 0, 3, 6, 12, 26, and 60 min after patch removal. During patching, they performed ordinary office tasks such as web browsing. MD monocular deprivation, NDE non-dominant eye, AE amblyopic eye
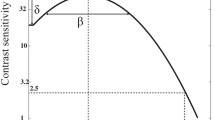
A binocular phase combination task. Two separate sinusoidal gratings were presented to the two eyes. These gratings had opposite phase shifts (+ 22.5° or − 22.5°) relative to the center of the screen. In this example, the phase shift is negative for the fellow/dominant (non-patched) eye and positive for the non-dominant/amblyopic eye (patched). If the patched (non-dominant/amblyopic) eye gets stronger, the perceived phase in the fused stimuli will become positive. This figure has been adapted from Min et al. [38]. NDE non-dominant eye, AE amblyopic eye, DE dominant eye, FE fellow eye
Apparatus
We programmed the experiment with MATLAB 2012a and PsychToolBox 3.0.9 extensions. We measured sensory eye balance of all subjects on a Mac computer by presenting dichoptic stimuli with gamma-corrected head mount goggles (NED Optics Groove pro, OLED), which have a refresh rate of 60 Hz and resolution of 1920 × 1080 to each eye. The maximal luminance of the goggles was 150 cd/m2.
Binocular Phase Combination Task
In this study, we used a binocular phase combination task [40] to measure binocular balance of each observer (Fig. 2). Two separate horizontal sine-wave gratings (spatial frequency = 0.46 cycles/deg, size = 4.33° × 4.33°) with equal and opposite phase shifts (+ 22.5° and − 22.5°) relative to the center of the screen were presented to both eyes. The perceived phase of fused stimuli was 0° when the two eyes contributed equally to binocular fusion. If the phase shift was negative for the fellow/dominant (non-patched) eye and positive for the non-dominant/amblyopic eye (patched), the perceived phase in the fused stimuli would become positive if the binocular balance was shifted in favor of the deprived (non-dominant or amblyopic) eye. A trial of the combination task had two phases: alignment and test phases. During the alignment phase, subjects were asked to align four dichoptic dots so that the distances between the neighboring dots were equal. This task ensured proper fusion throughout the measure. Then subjects began the test phase during which the two sinusoidal gratings were presented (Fig. 2). The subjects located their perceived middle portion of the dark patch in the fused grating by placing a flanking 1-pixel reference line. The stimuli were indefinitely displayed until subjects completed the tasks.
Throughout the task the amblyopic eye viewed the stimuli at a fixed contrast of 100%, whereas the fellow eye viewed them at a fixed contrast of 100% × balance point (δ). The balance point was defined as the value of the interocular contrast ratio between the non-patched eye (dominant/fellow eye) and the patched eye (non-dominant/amblyopic eye) when they contributed equally to binocular vision (Fig. 2). In other words, it refers to the contrast ratio where the two eyes contributed equally to binocular combination. The perceived phase would be 0° at the balance point. For each subject, the balance point (i.e., the contrast ratio) would be unique because their relative dominance of the one eye over another eye would differ. In all amblyopic observers, the relative contrast of the grating shown to the unpatched eye (i.e., fellow eye) was less than that to the patched eye (i.e., amblyopic eye). An individual’s balance point was used to set up the binocular perceived phase measures before and after patching. This selection of unique balance point enabled us to obtain a perceived phase of near 0 when each subject’s baseline was measured.
Two configurations of the stimuli were used to account for positional bias. For example, the dominant eye was once shown with a phase of − 22.5° and the amblyopic eye with a phase of + 22.5°, and then − 22.5° and + 22.5° respectively. We randomized the order of the configurations. This task has been shown to reliably measure changes in ocular dominance after patching with a low test–retest measurement variability [38]. For this reason, we speculate that repeated practice does not significantly affect the outcome measure itself (i.e., changes in perceived phase after patching relative to baseline).
Data Analysis
We used Python and R to analyze and visualize the data [41]. An analysis of variance (ANOVA) was used to identify factors, be it within- or between-subject, that significantly varied the data. Using the data of perceived phase (deg) as a function of time after deprivation (minutes), we computed the area (deg × minutes) under a curve (AUC) as an index for the longevity of the patching effect. Specifically, AUC was computed with the method of trapezoid integration (numpy.trapz function in NumPy from Python). Using the areal measures, we then performed post hoc analysis with a Tukey honestly significant difference (Tukey HSD) test and computed adjusted p values from pairwise comparisons. We also calculated the slope from linear regression between the perceived phase (y-axis) and timepoint (minutes, x-axis) to determine the recovery rate of the patching effect (i.e., rate of its decay). For slope analysis, ANOVA and Tukey HSD test were performed similarly. The alpha was established at 0.05 for statistical significance.
Results
Figure 3 illustrates how the shifts in sensory eye dominance persist over time after monocular deprivation in both normal and amblyopic observers. To begin with, we performed a one-sample t test to compare data of the perceived phase change (i.e., sensory changes in binocular balance) at 0 min after the deprivation with baseline (i.e., 0 in the y-axis of Fig. 3) to evaluate if deprivation at all durations introduced a significant shift in sensory eye balance. This analysis revealed a significant shift in both groups at all durations (p values < 0.05). Next, whether the magnitudes and longevities of the shifts differed between the two groups was assessed. First, we directly analyzed the data of changes in perceived phase (deg) across the observer group (between-subject factor), the timepoints after deprivation (within-subject factor), and the deprivation durations (within-subject factor) using a three-way mixed ANOVA (Fig. 3 and Supplementary Table). This analysis enabled us to examine whether the perceived phase significantly differed between the observer group, across timepoints after deprivation, and among the patching durations. Our analysis revealed that the effect of group was significant (F(1,22) = 4.44, p = 0.047, \({\eta }^{2}\) = 0.063), as well as the effects of timepoint (F(5,110) = 13.17, p < 0.001, \({\eta }^{2}\) = 0.083) and duration (F(2,44) = 17.53, p < 0.001, \({\eta }^{2}\) = 0.17). These results indicate that the magnitudes of the sensory visual change were significantly different between the two observer groups, across time after deprivation and among the patching durations. Moreover, the analysis showed that the interaction between the observer group and timepoint was statistically significant (F(5,110) = 9.54, p < 0.001, \({\eta }^{2}\) = 0.062), thereby illustrating that the decay rate of the patching effect could be significantly different between the observer groups; this speculation is confirmed with a linear slope analysis (Fig. 4). In other words, the magnitude of changes in binocular balance was much larger in normal observers but their longevity was significantly longer in the amblyopic group.
Averaged results across the amblyopic (n = 12, purple) and normal (n = 12, gray) observers. The plots illustrate the rate of the patching effect’s recovery (i.e., rate of decay). Each point denotes changes in binocular balance at each timepoint after the deprivation. Darker colors represent longer durations (ex. 300 min). Slopes that were computed with linear regression (x-axis in the linear scale) are in each panel. The error bars represent standard errors
Averaged rates of the patching effect’s recovery for each duration and the subject group. Gray bars represent data from the normal observers (n = 12), whereas the purple bars represent data from the amblyopic observers (n = 12). a Averaged recovery slopes from normal observers (n = 12). b Averaged recovery slopes from amblyopic observers (n = 12). The error bars represent standard errors of the slopes from all observers within the group. Asterisks denote a statistical significance from 0 based on a one-sample t test (*p < 0.05, **p < 0.01, ***p < 0.001)
To analyze the rate of the patching effect’s recovery (decay of its effect), we computed slopes from linear regression between changes in perceived phase (deg) and timepoints after deprivation (log10 of [minutes + 1]). We were keen to see if the recovery rate was significantly different between the two observer groups. According to a mixed ANOVA (within-subject factor, duration; between-subject factor, group), the slopes themselves were significantly different between the normal and amblyopic groups (F(1,22) = 11.28, p = 0.003, \({\eta }^{2}\) = 0.19). The slopes of amblyopic observers hovered around 0 (one-sample t test, p values > 0.09). Conversely, recovery slopes of normal observers at all three patching durations were significantly different from 0 (one-sample t test, p values < 0.01). Our results indicate that the decay rate of normal observers is more rapid as a function of patching duration than that of amblyopic observers.
In addition, to determine whether the slopes of normal observers were different among the patching durations, we used a one-way ANOVA (within-subject factor, patching duration). The analysis revealed that the recovery slopes did not significantly differ among the patching durations (F(2,22) = 0.70, p = 0.51, \({\eta }^{2}\) = 0.041). This finding indicates that the decay rate of the patching effect is similar across all patching durations in normal observers (Fig. 4a). In addition, a one-way ANOVA (within-subject factor, duration) indicated that the recovery rate was similar among all patching durations in amblyopic observers (F(2,22) = 0.87, p = 0.43, \({\eta }^{2}\) = 0.032; Fig. 4b). In other words, the recovery rate of the changes in binocular balance after inverse deprivation was found to be similar among the patching durations for each observer group.
An area under a curve (AUC) can capture both the magnitude and longevity of the shifts in sensory eye dominance after monocular deprivation as a single index. For this reason, we computed the AUC (as shown by the transparent filled colors in Fig. 3) for each observer group and patching duration. The computed areas of each subject and their averages per observer group are shown in Figs. 5 and 6 for normal and amblyopic observers, respectively.
Individual AUCs of normal observers (n = 12) for each patching duration. Each point represents the AUC for each subject at each duration. Darker points denote longer patching durations. Slopes that were computed with linear regression are shown in each panel. The error bars represent standard errors in the average plot
Individual AUCs of amblyopic observers (n = 12) for each patching duration. Each point represents the AUC for each subject at each duration. Darker points denote longer patching durations. Slopes that were computed with linear regression are in each panel. The error bars represent standard errors (barely visible) in the average plot
First, we wanted to examine whether the AUCs varied significantly between normal and amblyopic observers at each patching duration (30, 120, and 300 min; see Fig. 7). To do so, we performed a two-way mixed ANOVA (within-subject factor, patching duration; between-subject factor, subject group). The analysis indicated a statistically significant difference in the AUCs among the patching durations (F(2,44) = 18.34, p < 0.001, \({\eta }^{2}\) = 0.26) and between the groups (F(1,22) = 5.66, p = 0.27, \({\eta }^{2}\) = 0.13; Fig. 7). However, there was no significant interaction between the group and patching duration (F(2,44) = 2.33, p = 0.11, \({\eta }^{2}\) = 0.043). Our results illustrate that the AUCs are significantly different between the groups and among the patching durations for both observer groups.
Bar plots that show averaged AUCs of the normal (n = 12, gray) and amblyopic observers (n = 12, purple) for each patching duration. a Averaged areal measures from normal observers (n = 12). b Averaged areal measures from amblyopic observers (n = 12). Darker bars denote longer patching durations. The error bars represent standard errors
Moreover, to investigate whether the patching duration significantly affected the magnitude of neuroplastic changes in normal observers (Fig. 5), we performed a one-way ANOVA (within-factor, duration). The analysis revealed a statistically significant effect of patching duration (F(2,22) = 16.98, p < 0.001, \({\eta }^{2}\) = 0.36). This indicates that a large duration of patching induced a larger shift in binocular balance in normal observers (Fig. 5). A post hoc analysis using Tukey HSD test with a p value correction for multiple comparison showed that there was a significant difference of AUCs between 30 min and 120 min (p = 0.0078), but not between 120 min and 300 min (p = 0.38).
In addition, we wanted to examine if the patching duration significantly influenced the magnitude of the shift in binocular balance in amblyopic observers (Fig. 6). A one-way ANOVA (within-factor, duration) revealed a statistically significant effect of patching duration (F(2,22) = 5.28, p = 0.013, \({\eta }^{2}\) = 0.36). Post hoc analyses (Tukey HSD test with p value adjustment) revealed that there was a significant difference of AUCs between 30 min and 120 min (p = 0.048), but not between 120 min and 300 min (p = 0.99). This shows that a large duration of patching could induce a larger shift in binocular balance in amblyopic observers.
Furthermore, we performed a Pearson test to evaluate whether there was a significant difference between the difference in visual acuity and the AUCs from 300-min deprivation. There was no significant correlation between the two variables, suggesting that there is no clinical relationship between their visual acuity and the individual variability of the AUC (r = 0.31, p = 0.33).
The reader might notice from Fig. 7 that AUCs increase differently as a function of patching duration between normal and amblyopic observers. This may be because AUC of normal observers at 300-min duration seems to be much higher than at 120-min duration, whereas AUC at 300-min duration is quite similar to that at 120-min duraction in the amblyopic observers (Fig. 7). In other words, the dependence of the AUCs on the patching duration seems to be higher in the normal group than the amblyopic group. We directly computed the dependence (Fig. 8) by obtaining the slope from linear regression between AUC (deg × min) and patching duration (min). According to a one-sample t test, dependences of AUC on patching duration in both groups were found to be significantly different from 0 (p values < 0.05, Fig. 8). However, an unpaired two-sample Welch t test indicated that there was no statistically significant difference in the severity of the dependence between the groups (t(21.8) = 1.8, p = 0.076).
Bar plot that shows averaged slope of AUCs (deg × minutes) as a function of patching duration (minutes). The gray bar represents the slopes from the normal observers (n = 12), and the purple the amblyopic observers (n = 12). Asterisks denote a statistical significance from 0 based on a one-sample t test (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
Studies using various methods show that the visual input from the amblyopic eye is weighted less than that from the fellow eye during binocular fusion in both amblyopic and treated individuals [9, 10, 42,43,44,45,46,47,48], thereby illustrating an abnormal interaction between the amblyopic and fellow eyes. One mechanism for this phenomenon could be the more severe suppression from the fellow eye to the amblyopic eye. Therefore, it is of clinical interest to shift the sensory balance so that the input of the amblyopic eye gets weighted more by mitigating the abnormal suppressive interaction during binocular integration. One simple means to achieve this is by depriving the amblyopic eye; this protocol is known as inverse monocular deprivation. Recent clinical studies have established that inverse monocular deprivation could benefit both monocular and binocular visual functions for up to 1 year after the treatment, but whether there is an optimal duration remains unclear [32, 33]. Therefore, in this study, we investigated whether there would be a difference in shifts in sensory eye balance after monocular deprivation at different durations in both normal and amblyopic adults. Our results show that normal observers have a faster recovery rate of the sensory change as a function of patching duration than amblyopic observers. In fact, it seems to be near zero for the amblyopic observers (i.e., very little recovery over the testing period). These results indicate that the life span of the changes following short-term deprivation in the amblyopic observers seems to be relatively longer. However, it is important to note that the magnitude of changes (i.e., AUCs) in sensory eye dominance in normal observers is significantly larger than that of amblyopic counterparts. Therefore, it could appear that the amblyopic observers have less shifts that could be lost over time. Nevertheless, the changes after the deprivation in subjects with amblyopia were significantly different from their baseline. Taken together, the conclusion that the recovery rate is indeed slower in amblyopic observers seems to be sound since both observer groups exhibited a significant shift in binocular balance after monocular deprivation at all durations. In addition, we found that the ocular dominance changes depend on the duration of deprivation in both normal and amblyopic observers. However, a previous study reports only a very weak dependence on deprivation (statistically insignificant) duration for normal observers [35]. So, what factors could be responsible for the discrepancy?
Figure 9 shows a comparison between the previous and present data from normally sighted adults. The trend of the shift in eye dominance as a function of patching duration exists in both data sets. However, the trend in our previous data set does not reach statistical significance, possibly for two reasons (Fig. 9a). First, the magnitude changes are less (possibly due to the different method of measurement) and the data variability is greater. There could be a number of reasons for this. To begin with, the obvious difference is the sample size and experimental design between the current and previous studies. In the study by Min et al. [35], the sample size is smaller and the subjects that were recruited across different patching durations were not paired. This might have reduced the effect size and increased the variability between subjects. In the current study, a paired comparison design is used (Fig. 9b). Moreover, the visual task is different; the previous study uses a phase combination task that shows a reliable, but less sensitive, measurement of changes in sensory eye balance after monocular deprivation [38]. The current study, however, uses another combination task that is much more sensitive to the sensory change with a robust reliability [38]; this should have contributed to the increased magnitude of effect seen in the current study. Therefore, by using a reliable and sensitive visual test, we can report a significant effect of deprivation duration on the change in integrated visual plasticity in this study. Nonetheless, the strength of this dependence is weak even if it is captured by the more sensitive combination task. Across a tenfold change in deprivation duration, there is only a threefold change in visual plasticity in normally sighted observers from this study (Fig. 9b). In sum, both studies indicate that substantial increases in patching duration result in only slight changes in binocular balance.
Areal measures derived from post-patching data at 0 to 60 min after patch removal in normally sighted observers from a previous and current studies. a Integrated shifts in sensory eye dominance as measured with a binocular phase combination task at multiple contrasts [38] in seven observers after 30-min patching, eight observers after 120-min patching, and three observers after 300-min patching as shown in Min et al. [35]. The subjects from 30-min patching are not paired from the last two conditions. b Integrated shifts in sensory eye dominance as measured with a binocular phase combination task at a single contrast (see “Methods”) in 12 paired observers. The error bars represent standard errors
Another difference between the previous [35] and current studies is that the patched eye here is the non-dominant eye in normal observers, whereas it is the dominant eye in the previous study. This difference could be relevant to the discrepancy in the results between the previous and current studies. The primary visual cortex exhibits a homeostatic property of visual plasticity at the level of GABAergic inhibition [28]. The goal of the homeostasis mechanism is to keep the average neural activity constant by keeping the excitatory/inhibitory balance stable. Since short-term monocular deprivation strengthens the deprived eye’s contribution in binocular vision, depriving the dominant eye can further disrupt the homeostatic balance. Hence, the change after the deprivation is against the direction to which the homeostasis pushes for the intracortical balance between excitation and inhibition [22]. Hence, the potential for sensory change after depriving the dominant eye could be less as a result of the homeostasis [27]. On the other hand, if the non-dominant eye is deprived, the following changes are in accordance with the direction of the homeostasis because the increased contribution of the non-dominant eye can improve binocular balance. Therefore, the perceptual change that occurs after depriving the dominant eye could be less (or more short-lived) than depriving the non-dominant eye. Our findings showing a longer life span of the sensory changes in amblyopic observers than the normal counterparts after monocular deprivation support this notion and confirm an earlier report about amblyopia [29].
Our findings that the patching duration matters and that the longevity of the binocular benefit is more long-lasting in amblyopia could pave the road for both clinicians and researchers to better design patching protocols and provide a more effective binocular treatment for amblyopia. In future, it would be interesting to directly investigate the homeostatic nature of visual plasticity in adults with a neuroimaging or electrophysiological method. However, it is worth highlighting that the amblyopic observers in this study are all anisometropic and mostly exhibit a mild loss in stereopsis and visual acuity, thereby giving rise to the possibility that our results might not be generalizable to other types of amblyopia. Hence, future studies should also examine the effect of deprivation durations in other types of amblyopia using the same or other visual tasks that measure different levels of binocular functions, such as interocular suppression and binocular rivalry.
Relevance to Current Therapy for Amblyopia and Future Directions
The current treatment regimen for amblyopia involves depriving the fellow eye as an early intervention. However, this protocol has problems, such as a poor compliance rate [49], a high recurrence of amblyopia even after recovery [50, 51], and a poor binocular recovery. Inverse deprivation, as shown in this study, however patches the amblyopic eye, thereby allowing patients to see through their unaffected eye throughout the treatment. This could raise the compliance rate. In light of our results, 120-min inverse deprivation can be sufficient to induce maximal binocular benefits because our data indicate that the shifts in eye dominance are comparable between 120- and 300-min patching.
To ascertain whether inverse patching can be applied in the clinic, one has to evaluate the relative importance of monocular and binocular visual improvements to the everyday visual function and whether any benefit persists long after the treatment period [51]. Two recent studies highlight that inverse patching therapy can be effective for adult patients. Both show that these visual improvements can be sustained for up to 1 year after the treatment [32, 33]. A future study should directly investigate the effects of longitudinal inverse deprivation on various visual functions, such as interocular suppression and monocular contrast sensitivity of the deprived eye, stereopsis, and whether changes or improvements in these visual functions following inverse patching persist [13, 52,53,54].
Strengths and Weaknesses of the Visual Test in this Study
We used a binocular combination task using a single contrast ratio between the eyes at a low spatial frequency (0.46 cycles/deg) and a high suprathreshold contrast. This choice of stimulus has a number of strengths. These stimuli are very visible to the amblyopic eye as the contrast sensitivity deficit is almost exclusively restricted to high spatial frequencies [55,56,57]. Moreover, the measurement error is reduced if a low spatial frequency is used in a phase task for subjects with amblyopia [43]. Also, amblyopic phase discrimination is normal in this spatial range [58,59,60,61]. In addition, studies show that subjects with amblyopia can binocularly fuse when suprathreshold stimuli are present [11]. Finally, it has recently been shown that interocular suppression in amblyopia is maximal at lower spatial frequencies for stimuli of the same suprathreshold contrast [62]. More importantly, this approach has been shown to be very sensitive to changes in binocular balance after short-term monocular deprivation in normally sighted adults [38].
For these reasons, we used a low spatial frequency stimulus. On the other hand, one weakness of this approach is that the results that we report here at low spatial frequencies may not be generalized to higher spatial frequency stimuli, where it would be problematic to use the current measurement approach. Recent studies have shown that binocular imbalance is more severely disrupted at high spatial frequency in subjects with amblyopia [10, 46, 48, 63, 64], so it is not to be assumed that short-term occlusion will affect balance at all spatial frequencies equally. Future studies might be able to answer this question at high spatial frequencies using a different measurement approach, e.g., our recently developed orientation combination approach [46, 65].
Conclusions
In this study, the effect of patching duration was re-examined in detail by recruiting both normal and amblyopic adults and employing a paired experimental design. We deprived their non-dominant (or amblyopic) eye for 30, 120, and 300 min and observed whether the magnitude of subsequent shifts in sensory balance differed among the durations. We found a weak, but statistically significant, relationship between patching duration and the following shifts in both normal and amblyopic observers.
References
Daw NW, Daw NW. Visual development, vol. 14. New York: Springer; 2006.
Levi DM, Carkeet AD. Amblyopia: a consequence of abnormal visual development. In: Simons K, editor. Early visual development, normal and abnormal. New York: Oxford University Press; 1993; p. 391–408.
Kiorpes L, Daw N. Cortical correlates of amblyopia. Vis Neurosci. 2018. https://doi.org/10.1017/S0952523817000232.
Birch EE, Kelly KR, Wang J. Recent advances in screening and treatment for amblyopia. Ophthalmol Ther. 2021;10(4):815–30.
Kanonidou E. Amblyopia: a mini review of the literature. Int Ophthalmol. 2011;31(3):249–56.
Levi D, Harwerth R, Smith E. Binocular interactions in normal and anomalous binocular vision. Doc Ophthalmol. 1980;49(2):303–24.
Blakemore C. The conditions required for the maintenance of binocularity in the kitten’s visual cortex. J Physiol. 1976;261(2):423–44.
Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt. 2014;34(2):146–62.
Huang CB, Zhou J, Lu ZL, Feng L, Zhou Y. Binocular combination in anisometropic amblyopia. J Vis. 2009;9(3):17–17.
Kwon M, Wiecek E, Dakin SC, Bex PJ. Spatial-frequency dependent binocular imbalance in amblyopia. Sci Rep. 2015;5(1):1–12.
Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007;48(11):5332–8.
Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: a mini-review. Vision Res. 2015;114:17–30.
Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res. 2015;114:4–16.
Levi DM. Pathophysiology of binocular vision and amblyopia. Curr Opin Ophthalmol. 1994;5(5):3–10.
Schor C. A directional impairment of eye movement control in strabismus amblyopia. Invest Ophthalmol Vis Sci. 1975;14(9):692–7.
Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye–hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci. 2011;52(3):1851–64.
Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J Am Assoc Pediatric Ophthalmol Strabismus. 2015;19(6):515–20.
Kelly KR, Jost RM, De La Cruz A, et al. Slow reading in children with anisometropic amblyopia is associated with fixation instability and increased saccades. J Am Assoc Pediatric Ophthalmol Strabismus. 2017;21(6):447–51.
Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception in children aged 3 to 7 years with amblyopia and its association with deficits in vision and fine motor skills. JAMA Ophthalmol. 2019;137(5):499–506.
Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol. 2019;137(2):167–74.
Loudon S, Simonsz H. The history of the treatment of amblyopia. Strabismus. 2005;13(2):93–106.
De Buffon C. Disseration sur la cause du strabisme ou des yeux louches. Me Acad R Sci. 1743;1743:1743.
Mezad-Koursh D, Rosenblatt A, Newman H, Stolovitch C. Home use of binocular dichoptic video content device for treatment of amblyopia: a pilot study. J Am Assoc Pediatric Ophthalmol Strabismus. 2018;22(2):134–8.
Vedamurthy I, Nahum M, Huang SJ, et al. A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision Res. 2015;114:173–87.
Lunghi C, Burr DC, Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr Biol. 2011;21(14):R538–9.
Zhou J, Clavagnier S, Hess RF. Short-term monocular deprivation strengthens the patched eye’s contribution to binocular combination. J Vis. 2013;13(5):12–12.
Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107.
Lunghi C, Berchicci M, Morrone MC, Di Russo F. Short-term monocular deprivation alters early components of visual evoked potentials. J Physiol. 2015;593(19):4361–72.
Zhou J, Thompson B, Hess RF. A new form of rapid binocular plasticity in adult with amblyopia. Sci Rep. 2013;3(1):1–5.
Wallace MP, Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Compliance with occlusion therapy for childhood amblyopia. Invest Ophthalmol Vis Sci. 2013;54(9):6158–66.
Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33:67–84.
Lunghi C, Sframeli AT, Lepri A, et al. A new counterintuitive training for adult amblyopia. Ann Clin Transl Neurol. 2019;6(2):274–84.
Zhou J, He Z, Wu Y, et al. Inverse occlusion: a binocularly motivated treatment for amblyopia. Neural Plast. 2019;2019:1–12.
Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1402–8.
Min SH, Baldwin AS, Reynaud A, Hess RF. The shift in ocular dominance from short-term monocular deprivation exhibits no dependence on duration of deprivation. Sci Rep. 2018;8(1):1–9.
Ramamurthy M, Blaser E. The ups and downs of sensory eye balance: monocular deprivation has a biphasic effect on interocular dominance. Vision Res. 2021;183:53–60.
Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci. 2008;105(10):4068–73.
Min SH, Gong L, Baldwin AS, et al. Some psychophysical tasks measure ocular dominance plasticity more reliably than others. J Vis. 2021;21(8):20–20.
Berens C, Zerbe J. A new pinhole test and eye-dominance tester. Am J Ophthalmol. 1953;36(7 1):980–1.
Ding J, Sperling G. A gain-control theory of binocular combination. Proc Natl Acad Sci. 2006;103(4):1141–6.
Min SH, Zhou J. smplot: an R package for easy and elegant data visualization. Front Genet. 2021. https://doi.org/10.3389/fgene.2021.802894.
Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013;23(8):R308–9.
Kwon M, Lu ZL, Miller A, Kazlas M, Hunter DG, Bex PJ. Assessing binocular interaction in amblyopia and its clinical feasibility. PLoS ONE. 2014;9(6): e100156.
Li J, Hess RF, Chan LY, et al. Quantitative measurement of interocular suppression in anisometropic amblyopia: a case-control study. Ophthalmology. 2013;120(8):1672–80.
Hess R, Hutchinson C, Ledgeway T, Mansouri B. Binocular influences on global motion processing in the human visual system. Vision Res. 2007;47(12):1682–92.
Min SH, Mao Y, Chen S, He Z, Hess RF, Zhou J. A clinically convenient test to measure binocular balance across spatial frequency in amblyopia. iScience. 2022;25(1): 103652.
Min SH, Mao Y, Chen S, Hess RF, Zhou J. Modulation of mean luminance improves binocular balance across spatial frequencies in amblyopia. iScience. 2022;25:104598.
Chen S, Min SH, Cheng Z, et al. Binocular visual deficits at mid to high spatial frequency in treated amblyopes. iScience. 2021;24(7): 102727.
Dixon-Woods M, Awan M, Gottlob I. Why is compliance with occlusion therapy for amblyopia so hard? A qualitative study. Arch Dis Child. 2006;91(6):491–4.
Holmes JM, Melia M, Bradfield YS, Cruz OA, Forbes B, Pediatric Eye Disease Investigator Group. Factors associated with recurrence of amblyopia on cessation of patching. Ophthalmology. 2007;114(8):1427–32.
Hess RF. Reasons why we might want to question the use of patching to treat amblyopia as well as the reliance on visual acuity as the primary outcome measure. BMJ Open Ophthalmol. 2022;7(1): e000914.
Li SL, Reynaud A, Hess RF, et al. Dichoptic movie viewing treats childhood amblyopia. J Am Assoc Pediatric Ophthalmol Strabismus. 2015;19(5):401–5.
Xiao S, Angjeli E, Wu HC, et al. Randomized controlled trial of a dichoptic digital therapeutic for amblyopia. Ophthalmology. 2022;129(1):77–85.
Min SH, Chen S, Xu J, et al. A randomized clinical trial comparing eyetronix flicker glass and patching for treatment of amblyopia in children reveals similar improvements in vision. Front Neurosci. 2021;15: 622729.
Gstalder RJ, Green DG. Laser interferometric acuity in amblyopia. J Pediatr Ophthalmol Strabismus. 1971;8(4):251–6.
Hess R, Howell E. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977;17(9):1049–55.
Levi D, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Invest Ophthalmol Vis Sci. 1977;16(1):90–5.
Barrett BT, Pacey IE, Bradley A, Thibos LN, Morrill P. Nonveridical visual perception in human amblyopia. Invest Ophthalmol Vis Sci. 2003;44(4):1555–67.
Hess RF, Malin SA. Threshold vision in amblyopia: orientation and phase. Invest Ophthalmol Vis Sci. 2003;44(11):4762–71.
Lawden M, Hess RF, Campbell F. The discriminability of spatial phase relationships in amblyopia. Vision Res. 1982;22(8):1005–16.
Levi DM, Pass AF, Manny RE. Binocular interactions in normal and anomalous binocular vision: effects of flicker. Br J Ophthalmol. 1982;66(1):57–63.
Zhou J, Reynaud A, Yao Z, et al. Amblyopic suppression: passive attenuation, enhanced dichoptic masking by the fellow eye or reduced dichoptic masking by the amblyopic eye? Invest Ophthalmol Vis Sci. 2018;59(10):4190–7.
Ding J, Klein SA, Levi DM. Binocular combination in abnormal binocular vision. J Vis. 2013;13(2):14–14.
Mao Y, Min SH, Chen S, et al. Binocular imbalance in amblyopia depends on spatial frequency in binocular combination. Invest Ophthalmol Vis Sci. 2020;61(8):7–7.
Wang Y, He Z, Liang Y, et al. The binocular balance at high spatial frequencies as revealed by the binocular orientation combination task. Front Hum Neurosci. 2019;13:106.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China Grant (NSFC 31970975), the Natural Science Foundation for Distinguished Young Scholars of Zhejiang Province, China (LR22H120001), the National Key Research and Development Program of China Grant (2020YFC2003800) and the Project of State Key Laboratory of Ophthalmology, Optometry and Vision Science, Wenzhou Medical University (no. J02-20210203) to JZ, the Zhejiang Basic Public Welfare Project (LGJ20H120001) to ZH, Canadian Institutes of Health Research Grants CCI-125686 and 228103, and an ERA-NET Neuron grant (JTC2015) to RFH, and a doctoral award from the Canadian Institutes of Health Research to SM. The journal’s Rapid Service Fees were funded by the authors.
Authorship
All authors in this report meet the authorship criteria from the International Committee of Medical Journal Editors (ICMJE), take full responsibility for the entire work and approved the final version of the manuscript.
Author Contributions
Seung Hyun Min, Yiya Chen, Zhifen He, Robert F Hess and Jiawei Zhou designed the study. Yiya Chen and Nan Jiang collected the data. Seung Hyun Min, Yiya Chen, Robert F Hess and Jiawei Zhou analyzed the data. Seung Hyun Min and Robert F Hess wrote the manuscript. All authors revised and approved the submitted manuscript.
Disclosures
Seung Hyun Min, Yiya Chen, Nan Jiang, Zhifen He, Jiawei Zhou and Robert F Hess confirm that they have no conflicts of interest to declare.
Compliance with Ethics Guideline
This study is in line with the Declaration of Helsinki and was approved by the Institutional Review Boards at McGill University and Wenzhou Medical University.
Data Availability
The data can be shared from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Min, S.H., Chen, Y., Jiang, N. et al. Issues Revisited: Shifts in Binocular Balance Depend on the Deprivation Duration in Normal and Amblyopic Adults. Ophthalmol Ther 11, 2027–2044 (2022). https://doi.org/10.1007/s40123-022-00560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00560-5