Abstract
Immune checkpoint inhibitors (ICIs) have revolutionised the field of oncology. While most ICIs are well-tolerated, severe and fatal immune-related adverse events (irAEs) have been documented, likely related to the strengthened immunity harnessed by ICIs against tumours. Endocrinopathies are some of the most common irAEs, with both hypothyroidism and hyperthyroidism encountered after ICI use. As such, patients with pre-existing autoimmune conditions, such as Graves’ disease (GD) with clinically active thyroid eye disease (TED), are excluded from most clinical trials studying ICIs due to concerns of exacerbating pre-existing autoimmune conditions or of increasing the potential for irAE development. The limited information currently available on the safety and efficacy of ICIs in this population poses a clinical challenge for oncologists. The objective of this commentary is to highlight these challenges and provide treatment recommendations pertaining to two specific cohorts of patients with GD, namely GD patients with minimal eye complications and GD patients with previous TED who underwent radiotherapy, surgery or pulse methylprednisolone and whose disease is now quiescent, and to patients with subclinical autoimmune thyroid disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immune checkpoint inhibitors (ICIs) harness the body’s immune system against tumours. Immune-related adverse events (irAEs) are a side effect of this mechanism of action. |
Endocrinopathies are some of the most common irAEs The occurrence of irAEs are more common in patients with pre-existing autoimmune diseases (ADs) than in patients without. |
Use of immunosuppressants 2–4 weeks prior to and throughout ICI treatment has been proven to be effective in preventing exacerbations of pre-existing ADs. |
A personalised approach in the use of ICIs is thus required and highlights the importance of inter-disciplinary collaboration between clinical oncology and ophthalmology. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13090259.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionised the field of oncology. By blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) pathways, ICIs have benefited patients with metastatic cancers in terms of significantly longer progression-free and overall survival [1,2,3]. However, while ICIs are mostly well-tolerated, severe and fatal adverse events have been documented. These immune-related adverse events (irAEs) are likely related to the strengthened immunity harnessed by ICIs against tumours. Endocrinopathies are some of the most common irAEs, with both hypothyroidism and hyperthyroidism encountered after ICI use [4]. The clinical manifestations of thyroid-related irAEs closely resemble autoimmune diseases but without the chronicity [5]. As such, patients with pre-existing autoimmune conditions, such as Graves’ disease (GD) with clinically active thyroid eye disease (TED), are excluded from most clinical trials studying ICIs due to concerns of exacerbating pre-existing autoimmune conditions or of increasing the potential of irAE development [6]. The limited information currently available on the safety and efficacy of ICIs in this population poses a clinical challenge for oncologists. The objective of this commentary is to highlight the challenges and provide recommendations pertaining to two specific cohorts of patients with GD, namely GD patients with minimal eye complications and GD patients with previous TED who underwent radiotherapy, surgery or pulse methylprednisolone (PMP) whose disease is now quiescent.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Graves’ Disease and Thyroid Eye Disease
Immunopathogenesis
Autoimmunity is characterised by a failure in either central or peripheral tolerance. Central tolerance refers to the elimination of T-cell receptors with high complementarity and affinity to self-peptides on major histocompatibility complexes (MHC) in the thymus [7]. T cells with weak affinity are positively selected and further develop into CD8 T cells or CD4 T cells depending on the receptors’ specificity to MHC Class I or II molecules, respectively. In the case of fallible self–nonself discrimination in the thymus, various immune checkpoint pathways regulate T-cell activation. Crucial to this process of peripheral tolerance are the CTLA-4 and PD-1 immune checkpoint pathways. Both act as a negative costimulatory signal to inhibit T-cell activity at different phases of activation (priming vs. effector) and at different anatomical locations [7]. CTLA-4 on activated T cells and regulator T cells (Treg) mediate immunosuppression through direct and indirect downregulation pathways. The direct interaction between CTLA-4 and B7 ligands on the surface of antigen-presenting cells induces T-cell anergy [8]. By binding with much higher affinity than and outcompeting the costimulatory receptor CD28, CTLA-4 indirectly increases the activation threshold of T cells and dampens immune responses against self-antigens [9, 10]. In comparison, PD-1, which is similar to CTLA-4 in structure and binding, antagonises TCR-CD28 signals via PD-1–PD-1L interactions [11], leading to a reduction in T-cell proliferation and survival, as well as cytokine production [7].
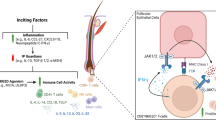
GD is characterised by the loss of tolerance to self-antigens in T cells, most commonly the thyroid-stimulating hormone receptor (TSHR). The activated helper T cells and their subsequent production of cytokines (interleukin [IL]-2, -4, -5) stimulate B cells to produce TSHR autoantibodies, which bind to and mimic the effect of thyroid hormone on the thyrotropin receptors. TED is an ocular inflammatory disease commonly associated with GD in which TSHR is also the primary autoantigen [12]. Clinically identifiable TED occurs in 40% of patients with GD, with common orbital and periorbital manifestations being eyelid retraction, proptosis, diplopia and increased intraocular pressure [13]. TED typically follows a biphasic course, with an initial active inflammatory phase and an ensuing static fibrotic phase [14]. The first phase reflects inflammatory changes induced by the activation of orbital fibroblasts by the TSHR autoantibodies. The release of proinflammatory mediators, compounded by the infiltration and interaction of immunocompetent T helper (Th1) and B lymphocytes, macrophages and mast cells, contribute to the enhanced adipogenesis that interferes with orbital functions [15].
Conventional Treatments for Thyroid Eye Disease
Treatment of TED requires a multidisciplinary effort from ophthalmologists, endocrinologists and radiologists. The primary goal is to restore a euthyroid state and to prevent sight-threatening ocular complications.
Treatment modalities for TED depend on the severity and activity of the disease. For mild TED, local supportive measures are the mainstay treatment. In one randomised controlled trial (RCT), selenium supplementation was shown to improve ocular parameters and quality of life in patients with mild TED when compared with placebo [16]; however, the lack of data on serum selenium levels and the possibility of marginal selenium deficiency in the patients tested may have skewed the data. For patients with moderate and severe TED, current treatment strategies focus on immunosuppression in the active phase. Systemic glucocorticoids are regarded as the first-line treatment, with weekly intravenous pulse treatment being favoured over oral treatment due to its higher efficacy and fewer adverse effects [17, 18]. The improvement in ophthalmic symptoms is thought to be due to inhibited transcription of intra- and extracellular proinflammatory proteins in orbital fibroblasts and Th1 lymphocytes [15]. If the patient is intolerant or the TED is refractory to steroids, second-line medical therapies include combination therapy with steroid-sparing agents, such as cyclosporine and azathioprine.
These well-established treatment modalities do not minimise the likelihood of patients requiring subsequent rehabilitative surgery for residual disfigurements and dysfunction. Therefore, there is a need for more targeted therapeutics. Biological agents, such as rituximab (humanised chimeric monoclonal anti-CD 20 antibody), despite a compelling mechanism of action, have demonstrated conflicting results in two RCTs [19, 20]. However, it is worth noting that the small sample sizes and the differences in baseline parameters of the patients recruited to these two RCTs may account for the discrepancy. Results from small case studies have also supported the use of immunomodulatory agents targeting key cytokines—IL-1, IL-6 and tumour necrosis factor-alpha. A new targeted therapy involving teprotumumab, a fully human IgG1 monoclonal antibody directed against the insulin-like growth factor 1 receptor, has demonstrated promising results in reversing diplopia and proptosis in two multinational RCTs [21, 22], but the clinical use of this monoclonal antibody, especially in public healthcare systems, is limited by its high costs. Orbital radiotherapy is also indicated if there is involvement of extraocular muscle, with a modest effect on motility and proptosis [23], but surveys of European and American specialists indicate that this therapy is generally considered as a second- or third-line treatment [24, 25]. Acute side effects of orbital radiotherapy, such as periorbital oedema and conjunctival injection, are common and self-limiting [23], but long-term side effects, such as cataract, retinopathy and radiation-induced tumours post-radiotherapy, have been reported [23, 26].
For sight-threatening TED, including dysthyroid optic neuropathy, treatment is more aggressive, involving decompression through a combination of high-dose intravenous methylprednisolone and orbitotomy. Severe complications have been reported, which include acute liver damage, cardiac arrhythmias, hypertension, diabetes mellitus and osteoporosis following PMP and diplopia with orbital decompression [27,28,29].
Use of Immune Checkpoint Inhibitors and Thyroid Eye Disease-Like Immune-Related Adverse Events
Immune checkpoint inhibitors have emerged as a major pillar of modern cancer treatment. Neoplastic cells utilise physiological immune checkpoint pathways against autoimmunity to elude immunosurveillance. Through targeting the CTLA-4 and PD-1 immune checkpoint pathways, ICIs fundamentally upregulate the host’s immune system and mediate an antitumour response. Briefly, CTLA-4 blockade causes effector T cells at the priming phase to activate and proliferate, and reduces Treg-mediated suppression of T-cell responses [7, 30]. PD-1 blockade affects T cells at the effector phase and reverses T-cell exhaustion [31]. Since PD-L1 is expressed on cancer cells within the tumour microenvironment, PD-1 blockade confers a more specific and effective antitumour effect with less severe side effects [30]. Unfortunately, however, ICIs may be a double-edged sword. Tilting the balance of immune surveillance may perturb immunologic homeostasis. This is well-reflected by the emergence of irAEs. IrAEs have been reported in up to 80% of patients receiving these treatments, with a higher incidence in those receiving CTLA-4 inhibitors compared with PD-1 blockade [32]. While the exact pathophysiology of irAEs remains unclear, it is likely due to heightened T-cell activity against shared antigens, as well as elevated levels of inflammatory cytokines and pre-existing autoantibodies [33].
Ocular irAEs are rare but potentially deleterious. Dry eyes, uveitis and myasthenia gravis with ocular involvement are commonly reported ocular toxicities [34, 35]. TED-like orbital inflammatory syndrome has also been described in case studies [36,37,38,39]. Patients on anti-CTLA4 or anti-PD1 therapy with no history of thyroid disease have developed TED-related inflammatory symptoms with fusiform enlargement of rectus muscles involving muscle bodies and sparing the tendons. However, these patients had variable thyroid hormone profiles. Most were euthyroid with elevated thyroid antibodies (particularly anti-thyroid peroxidase and anti-thyroglobulin antibodies, occasionally anti-TSHR), and some patients had hyperthyroidism [36,37,38,39]. The majority of patients were managed successfully with systemic steroid treatment. Relapse and persistence of ocular symptoms after tapering of steroids has also been encountered [38]. The decision of whether/when to discontinue ICIs is not unanimous among clinicians, in part due to the concern that the systemic steroid may reverse the anti-tumour activity of ICIs.
The association between ICIs and TED may be related to allelic variants of the CTLA-4 and PD-1 genes. Genetic analyses over the last decade have established that CTLA-4 gene polymorphisms confer susceptibility to GD. Well-studied polymorphisms include an adenine-to-guanine single-nucleotide polymorphism (SNP) at position 49 in the CTLA-4 leader peptide (A/G49), a 3′ untranslated region (3′ UTR) microsatellite [(AT)n repeat] of exon 4 and a cytosine-to-thymine substitution at position -318 of the promotor region (C/T−318) [40]. The functional significance of these polymorphisms has been investigated in various case control studies, mainly relating to the reduced expression levels and function of CTLA4 [41,42,43] and production of thyroid autoantibodies (TAb) [40, 44]. Regarding the association of these genetic modifications with TED, a meta-analysis with 14 case–control studies demonstrated an increased risk of TED with the CTLA-4 A/G49 SNP in both Caucasian and Asian populations [45]. A case–control study on Taiwanese patients in 2019 identified the CTLA-4 promoter -1722 polymorphism, notably the TT genotype, and its relationship to an increased risk of TED [46]; however, these authors found no correlation between TED and the A/G49 SNP in exon 1.
The differences in genotypes and phenotypes may be accounted for by linkage disequilibrium, male-to-female ratio and the definition of TED [47]. Correlation studies on the polymorphisms and the molecular pathophysiology of TED have shown that the allelic variations in the promoter (-1722) and exon 1 (A/G49) reduce the transcription and expression of CTLA-4 [41, 48]. Since the exon 1 polymorphism (A/G49) also weakens the downstream signalling of CTLA-4, the aggregate effect reduces immune tolerance to self-antigens, namely TSHR. Correlations between TED and PD-1 genetic polymorphisms have also been identified in case–control studies. In a UK GD dataset of 2671 patients, two of the eight investigated tag SNPs were significantly associated with GD, but not with TED [49]. PD-1L A/C polymorphisms at position 8923 of intron 4 and their association with GD have also been demonstrated in Japanese and European patient cohorts [50, 51]. TED was used as one of the selection criteria for GD subjects in the European study, but the association between the polymorphism and TED was not explored [50].
The occurrence of irAEs are more common in patients with pre-existing autoimmune diseases (ADs) than in to patients without [6]. Yet, flares are mild to moderate in severity and are mostly controlled by standard treatment. ICI discontinuation is often not required, and efficacy of ICIs remain comparable between patients with and without pre-existing ADs. The pre-existing ADs that extant literature focus on are of a rheumatological (e.g. psoriasis, rheumatoid arthritis and systemic lupus erythematosus) and gastrointestinal nature (e.g. inflammatory bowel diseases) [6]; thyroid diseases are less common. This could reflect the prevalence of AD or the more severe or active presentation of these patients which deters oncologists from ICI use. Therefore, there remains a need to characterise patients with GD who may be eligible for ICIs. In this context, two specific cohorts of GD patients have been identified: (1) GD patients with minimal eye complications, and (2) GD patients with previous TED who underwent radiotherapy, surgery or PMP and whose disease is now quiescent.
It is important to recognise a third cohort of patients, those with subclinical autoimmune thyroid disorders. There remains uncertainty over the clinical utility of managing thyroid dysfunction in this patient population on the basis of biochemical derangements [52]. However, given the possibility of progression to overt disease, clinicians should also investigate these patients for parenteral and family anamnesis of autoimmune diseases. The limited data available from clinical trials on the aforementioned two patient populations highlight the need for high-quality clinical trials to focus on these cohorts. However, extrapolating and utilising existing recommendations for ICI administration in active pre-existing AD indicates that a personalised and stepwise strategy is optimal.
Personalised Strategy
It is important to recognise that the clinical manifestation of GD or TED is multifactorial and involves an intricate interaction between genetic, immunological and environmental factors, particularly smoking. Given the complexity of the tumour microenvironment and the dynamic interaction of inflammatory molecules in patients with autoimmune thyroid disorders, the choice of selective immunosuppressants may impact the efficacy of treatment. Anti-IL-6 receptor antibodies could preferentially be used in high-risk patients. IL-6, a paracrine and autocrine cytokine, has been speculated to be crucial in the pathophysiology of Graves’ ophthalmopathy, with higher serum concentrations in patients with active ophthalmopathy [53, 54]. The blockade of IL-6 and the PD-1 axis has been shown to have synergistic antitumour effects through the induction of cytotoxic T cells and the inhibition of in vivo tumourigenesis in animal studies [55, 56]. Anti-IL-6 receptor antibodies have also yielded rapid and sustained improvement in various ocular parameters in patients with refractory Graves’ orbitopathy [57]. A personalised strategy for patients highlights the importance of inter-disciplinary collaboration between clinical oncology and ophthalmology. An effective multidisciplinary clinical pathway for these patients should be established.
A Stepwise Approach
The chronology with which medications are given may render ICIs safe and effective. By replacing a non-specific immunosuppressant (e.g. steroids) with a selective immunosuppressant (e.g. infliximab, tocilizumab, vedolizumab) 2–4 weeks prior to and throughout ICI treatment, patients have shown optimal response with an absence of autoimmune disease exacerbations [6].
Conclusions
In conclusion, endocrinopathies are some of the most common irAEs. It is also important to note that patients with pre-existing GD are more likely to suffer from acute exacerbation of illness. Thus, a personalised approach in the use of ICIs in the context of GD and other autoimmune diseases is required. This highlights the importance of inter-disciplinary collaboration between clinical oncology and ophthalmology.
References
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82.
Hoefsmit EP, Rozeman EA, Haanen JBAG, Blank CU. Susceptible loci associated with autoimmune disease as potential biomarkers for checkpoint inhibitor-induced immune-related adverse events. ESMO Open. 2019;4(4):e000472.
Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020;31(6):724–44.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012;4(6):a006957.
Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8.
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–38.
Chin YH, Ng CH, Lee MH, et al. Prevalence of thyroid eye disease in Graves’ disease: a meta-analysis and systematic review. Clin Endocrinol. 2020. 10.1111/cen.14296.
Bartley GB. Rundle and his curve. Arch Ophthalmol. 2011;129(3):356–8.
Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye (Lond). 2013;27(3):308–19.
Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of Mild Graves’ orbitopathy. N Engl J Med. 2011;364(20):1920–31.
Zang S, Ponto KA, Kahaly GJ. Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320–32.
Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ orbitopathy guidelines for the management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5(1):9–26.
Salvi M, Vannucchi G, Beck-Peccoz P. Potential utility of rituximab for Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98(11):4291–9.
Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100(2):432–41.
Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–61.
Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–52.
Chundury RV, Weber AC, Perry JD. Orbital radiation therapy in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2016;32(2):83–9.
Perros P, Baldeschi L, Boboridis K, et al. A questionnaire survey on the management of Graves’ orbitopathy in Europe. Eur J Endocrinol. 2006;155(2):207–11.
Perumal B, Meyer DR. Treatment of severe thyroid eye disease: a survey of the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS). Ophthalmic Plast Reconstr Surg. 2015;31(2):127–31.
Zygulska A. Radiotherapy in the treatment of Graves ophthalmopathy—to do it or not? J Ocul Biol Dis Infor. 2009;3(1):1–11.
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid. 2007;17(4):357–62.
Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30.
Golan S, Gupta A, Goldberg RA. Double vision after minimally invasive orbital decompression. J Craniofac Surg. 2017;28(5):412–5.
Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9.
Eun Y, Kim IY, Sun J-M, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep. 2019;9(1):14039.
Connolly C, Bambhania K, Naidoo J. Immune-related adverse events: a case-based approach. Front Oncol. 2019;9:530.
Abdel-Rahman O, Oweira H, Petrausch U, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17(4):387–94.
Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–78.
Sagiv O, Kandl TJ, Thakar SD, et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast Reconstr Surg. 2019;35(1):50–2.
McElnea E, Ní Mhéalóid Á, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving ipilimumab. Orbit. 2014;33(6):424–7.
Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164(2):303–7.
Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg. 2011;27(4):e87–8.
Ban Y, Davies TF, Greenberg DA, et al. Analysis of the CTLA-4, CD28, and inducible costimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun. 2003;4(8):586–93.
Ligers A, Teleshova N, Masterman T, Huang WX, Hillert J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001;2(3):145–52.
Kouki T, Sawai Y, Gardine CA, Fisfalen M-E, Alegre M-L, DeGroot LJ. CTLA-4 gene polymorphism at position 49 in Exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves’ disease. J Immunol. 2000;165(11):6606.
Mäurer M, Loserth S, Kolb-Mäurer A, et al. A polymorphism in the human cytotoxic T-lymphocyte antigen 4 ( CTLA4) gene (exon 1 +49) alters T-cell activation. Immunogenetics. 2002;54(1):1–8.
Zaletel K, Krhin B, Gaberscek S, Pirnat E, Hojker S. The influence of the exon 1 polymorphism of the cytotoxic T lymphocyte antigen 4 gene on thyroid antibody production in patients with newly diagnosed Graves’ disease. Thyroid. 2002;12(5):373–6.
Wang H, Zhu L-S, Cheng J-W, et al. Meta-analysis of association between the +49A/G polymorphism of cytotoxic T-lymphocyte antigen-4 and thyroid associated ophthalmopathy. Curr Eye Res. 2015;40(12):1195–203.
Chen D-P, Chu Y-C, Wen Y-H, Lin W-T, Hour A-L, Wang W-T. Investigation of the correlation between Graves’ ophthalmopathy and CTLA4 gene polymorphism. J Clin Med. 2019;8:1842.
Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003;31(1):21–36.
Du P, Ma X, Wang C. Associations of CTLA4 gene polymorphisms with Graves’ ophthalmopathy: a meta-analysis. Int J Genom. 2014;2014:537969.
Newby PR, Roberts-Davies EL, Brand OJ, et al. Tag SNP screening of the PDCD1 gene for association with Graves’ disease. Clin Endocrinol (Oxf). 2007;67(1):125–8.
Mitchell AL, Cordell HJ, Soemedi R, et al. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab. 2009;94(12):5139–45.
Hayashi M, Kouki T, Takasu N, Sunagawa S, Komiya I. Association of an A/C single nucleotide polymorphism in programmed cell death-ligand 1 gene with Graves’ disease in Japanese patients. Eur J Endocrinol. 2008;158(6):817–22.
Wiersinga WM. Guidance in subclinical hyperthyroidism and subclinical hypothyroidism: are we making progress? Eur Thyroid J. 2015;4(3):143–8.
Molnár I, Balazs C. High circulating IL-6 level in Graves’ ophthalmopathy. Autoimmunity. 1997;25(2):91–6.
Salvi M, Pedrazzoni M, Girasole G, et al. Serum concentrations of proinflammatory cytokines in Graves’ disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol. 2000;143(2):197–202.
Ohno Y, Toyoshima Y, Yurino H, et al. Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci. 2017;108(10):1959–66.
Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–22.
Atienza-Mateo B, Calvo-Río V, Martín-Varillas JL, et al. SAT0601-anti-il6-receptor tocilizumab in graves’ orbitopathy multicenter study of 29 patients. Ann Rheum Dis. 2018;77(Suppl 2):1153.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Charlene YC Chau, Kendrick C Shih, Loraine LW Chow and Victor HF Lee were involved in study design, data collection, data analysis, manuscript writing and editing.
Disclosures
Charlene YC Chau, Kendrick C Shih, Loraine LW Chow and Victor HF Lee have nothing to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chau, C.Y.C., Shih, K.C., Chow, L.L.W. et al. Considerations for Use of Immune Checkpoint Inhibitors in Cancer Therapy for Patients with Co-Existing Thyroid Eye Disease. Ophthalmol Ther 10, 5–12 (2021). https://doi.org/10.1007/s40123-020-00317-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-020-00317-y