Abstract
Introduction
The aim of this study was to assess the incidence of persistent postoperative cystoid macular edema (pCME) in patients undergoing pars plana vitrectomy with epiretinal membrane peel (ERM) only versus those with ERM peel combined with internal limiting membrane peel (ILM). Secondary endpoints of the study were to review both the central macular thickness (CMT) and visual acuity.
Methods
The patients were divided in two groups, one group in which only the ERM was peeled (n = 36 patients) and another group in which both the ERM and the ILM were removed (n = 62 patients). The results were analyzed retrospectively. Each patient received a complete ophthalmological examination, including best-corrected visual acuity (BCVA) using an ETDRS chart and spectral domain optical coherence tomography, at three time points: prior to surgery and 3 weeks and 3 months after surgery.
Results
A total 98 eyes of 98 patients were included in this study. The mean follow-up time was 7.7 months. CMT decreased significantly after surgery in all patients, and none of these changes differed significantly between the two groups. The BCVA increased significantly after surgery across all patients, and there were no significant changes between the two treatment groups. Postoperative pCME occurred in eight patients in each group, representing 22.2% of the 36 patients in the ERM only group and 12.9% of the 62 patients in the ERM/ILM peel group. However, this difference was not statistically significant.
Conclusions
No difference was found between the two groups in terms of incidence of pCME. Both groups experienced had similar decrease in the CMT and improvement in the BCVA postoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An epiretinal membrane (ERM) is a fibrocellular tissue found on the inner surface of the retina. It is semi-translucent and forms on the surface of the internal limiting membrane (ILM). The most common form of ERM is idiopathic and occurs in elderly healthy eyes without any other apparent disease. However, retinal breaks/detachment, retinopexy and inflammation can also lead to secondary ERM formation [1]. The ILM is a transparent structure that defines the boundary between the retina and the vitreous and is the surface upon which the ERM develops. As the outer portion of the ILM is composed of the footplates of the Muller cells, the ILM acts as a rigid scaffold that transmits the distortion caused by the ERM onto the more flexible underlying retina. In this context, the ILM may play a key role in the pathophysiology of retinal disorders involving the vitreomacular interface [2].
In general, patients with symptomatic ERM complain of reduced and distorted vision. The current treatment of choice for symptomatic patients with an ERM is pars plana vitrectomy (PPV) with ERM peel, although in the first decade of this century ERM peel combined with removal of ILM was also routinely practiced during during surgery on the ERM with good anatomical results [3,4,5,6]. However, more recent studies have shown that ILM peeling is a procedure that can cause immediate traumatic effects and progressive modification of the underlying inner retinal layers [7,8,9,10,11,12]. In addition, it is controversial whether ILM peeling improves vision postoperatively faster or reduces the risk of postoperative persistent CME (pCME) [13, 14].
The aim of this study was to compare the outcomes after ERM surgery with or without ILM peeling in terms of vision, decrease in central macular thickness (CMT) and complication rates, in particular the occurrence rate of pCME.
Methods
All procedures followed were in accordance with the ethical standards of the institutional and/or national research committee (NHS Health Research Authority Bristol) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
All patients who underwent PPV for ERM at the Bristol Eye Hospital were identified and their case notes reviewed retrospectively. Patient with all forms of secondary ERM were excluded. Each patient received a complete ophthalmological examination, including best-corrected visual acuity (BCVA) using an Early Treatment Diabetic Retinopathy Study (ETDRS) chart (in letters) and spectral domain–optical coherence tomography (OCT) (Spectralis HRA OCT; Heidelberg Engineering, Heidelberg, Germany), at three time points: prior to surgery and at 3 weeks and 3 months after surgery.
Surgical Procedure
Surgery was performed only in symptomatic patients. A standard 3-port PPV (25-gauge) (Constellation Vision System; Alcon Inc., Fort Worth, TX, USA) was performed in all patients. Dual blue dye (Geuder AG, Heidelberg, Germany) was then slowly injected towards the macular area to stain the ERM and ILM. In the case of ILM peel, a second injection of Dual blue dye was performed after the ERM had been removed. A diamond brush was used to mobilize the ILM. Both ERM and ILM peels were performed in a circumferential pattern for about 1.5–2 disk diameters around the fovea using a 25G Eckardt end-gripping forceps. Patients had an air-filled eye at the end of the surgery. In the case of advanced cataract, a combined procedure was performed with a simultaneous phacoemulsification. In some cases 0.05 ml of triamcinolone acetate 40 mg/ml was injected intravitreally at the end of surgery. All procedures were performed by a team of four consultants and two experienced fellows.
Patients were examined postoperative day 1 and the intraocular pressure was checked. Further follow-ups with an OCT were performed 3 weeks and 3 months postoperatively. The visual acuity was tested using an ETDRS chart and recorded in letters.
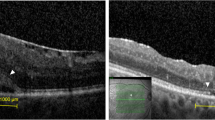
CMT was defined as the highest value on the standard Spectralis SD-OCT retinal thickness map. Persistent macular edema was defined as persistent intraretinal cysts on OCT images that remained for > 3 months postoperatively.
Data Analysis
Preoperative values of CMT and BCVA were compared between the two treatment groups (ERM only group; ERM/ILM peel group) using two-sample t tests. Changes after surgery were determined as the difference between preoperative values and postoperative values at 3 weeks, 3 months and last follow-up, respectively. We used one-sample t tests to determine whether the mean change (for all patients) differed from zero, and two-sample t tests to compare the two treatment groups. To account for multiple testing, we report Bonferroni–Holm-adjusted p values for the three postoperative time points. The frequency of postoperative CME was compared between treatment groups with a Chi-square test. Data analysis was performed with the statistical software R, version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total 98 eyes of 98 patients were included this study. The patients were divided in two treatment groups: in one group only the ERM was peeled (ERM only group, n = 36 patients), and in the other group both the ERM and the ILM were removed (IERM/LM peel group, n = 62 patients). The mean follow-up time was 7.7 months. A combined procedure with cataract surgery was performed in 41 patients, of whom 15 (42%) were in the ERM only group and 26 (42%) were in the ERM/ILM peel group. At the time of the operation, five (14%) patients in the ERM only group and 12 (19%) patients in the ERM/ILM peel group received an intravitreal injection of triamcinolone acetate (ivTA). Patients’ characteristics at baseline are summarized in Table 1.
Postoperative pCME occurred in eight patients in each group, representing 22.2% of the 36 patients in the ERM only group and 12.9% of the 62 patients in the ERM/ILM peel group. This difference was not statistically significant (Chi-square 1.45, p = 0.23). None of the 17 patients who were administered an ivTA (0%) developed pCME, whereas 16 of the 81 patients who were not administered an ivTA (19.8%) developed pCME; this difference was marginally significant (Chi-square 4.01, p = 0.045). The proportion of patients developing pCME did not differ significantly between those receiving combined phaco-vitrectomy, of whom 19.5% had pCME, and the patients who had no or previously performed cataract surgery, of whom 14% had pCME (Chi-square 0.52, p = 0.47).
Mean CMT did not differ significantly between the two groups preoperatively (473 [ERM only] vs. 465 µm [ERM/ILM peel], t = 0.45, p = 0.70). CMT decreased significantly after surgery in all patients, with a mean decrease of 63.8 µm after 3 weeks (95% confidence interval [CI] 48.4–79.1 µm, t = 8.3, adjusted p < 0.001), of 91.9 µm within 3 months (95% CI 72.6–111.2 µm, t = 9.5, adjusted p < 0.001) and of 107 µm by the last follow-up (95% CI 90.3–123.7 µm, t = 13.0, adjusted p < 0.001). None of these changes differed significantly between the two groups at these three time points (adjusted p = 0.33, 1.00 and 1.00, respectively).
Mean BCVA also did not differ significantly between the two groups preoperatively (55.9 [ERM only] vs. 59.6 letters [ERM/ILM peel], t = 1.7, p = 0.10). The postoperative BCVA increased significantly in all patients, with a mean increase of 4.4 letters after 3 weeks (95% CI 2.0–6.9, t = 3.6, adjusted p < 0.001), of 6.8 letters within 3 months (95% CI 4.1–9.4, t = 5.1, adjusted p < 0.001) and of 9.1 letters at the last follow-up (95% CI 6.4–11.9, t = 6.6, adjusted p < 0.001). Again, none of these changes differed significantly between the two treatment groups (adjusted p = 1.00 at each follow-up) (Fig. 1, 2). The mean improvement in BCVA at the three follow-up time points (3 weeks, within 3 months, last follow-up) did not differ significantly between patients receiving combined phaco-vitrectomy and vitrectomy only patients (adjusted p = 0.32, 1.00 and 1.00, respectively).
Mean change in central retinal thickness (CMT) and best-corrected visual acuity (BVCA) in the two treatment groups, measured for each person as the difference from the preoperative value. Error bars represent the standard error of the mean value (SEM). CMT Central macular thickness, ILM inner limiting membrane, OCT optical coherence tomography
Not one single patient had to be re-peeled due to the re-occurrence of ERM within the follow-up time.
Discussion
Until very recently the ILM was considered to be an integral part of the retina, and vitreoretinal surgeons did not think that it could be removed without causing visual damage. However, the publication of a number of primary reports of cases of spontaneous separation of the ILM, which resulted in no significant fibrosis and good visual acuity after surgery, has driven a change in the opinion of vitreoretinal surgeons on the possibility of removing the ILM to release vitreoretinal tractions [15, 16]. This change is also driven by additional findings. Histological studies on the removed ERM showed that, in up to 60% of patients, the ILM and ERM were removed together at the same time [17, 18]. It has also been reported that ILM specimens that were removed after ERM peeling demonstrated the presence of microscopic ERM remnants that persisted over the ILM in almost one-half of the patients; these ERM remnants could ultimately form islands of re-proliferation [4, 18, 19, 20]. Thus, ILM removal provides the certainty of having removed all cells that produce collagen above the retina. Additionally, this procedure ensures that all adhesions distorting the inner retina have been released. Therefore, the simultaneous removal of ERM and ILM has become a widely approved procedure in vitreoretinal surgery. Since the early 1990s, numerous studies have confirmed that ILM removal is associated with better anatomical improvement, better final vision outcome and lower risk of recurrence [3,4,5,6]. In one study, ILM peeling was also found to be the superior surgical approach to resolving cystoid macular edema due to less epiretinal traction, which disappeared in 90% of the patients, compared to 44% of patients who underwent removal of the ERM alone [3]. However, many other comparison studies found no functional difference between both groups [13, 14]. In addition, one retrospective study found ILM removal was correlated with worse visual outcome [21]. Tadayoni et al. first reported anatomical damage after ILM peeling and described a peculiar macular appearance, called “dissociated optic nerve fibre layer” (DONFL), which appears 1–3 months after surgery and leads to a thinning of the inner retina, causing a reduction in visual outcome [11]. Spaide et al. later clarified that DONFL corresponds to inner retinal dimples that course along the path of the nerve fiber layer. The dimples seem to be the result of an interplay between trauma and healing processes constrained by nerve fiber layer and do not appear to be due to any dissociation of optic nerve fibers. Furthermore, thickening of the macula without foveal depression has been found in 84% of eyes of ILM-peeled eyes, compared to 43% of eyes with unpeeled ILMs [10]. The authors of that study hypothesized that ILM peeling could damage the Müller cell footplates which form the inverted cone scaffold that gives the navel shape to the fovea.
Conclusions
In conclusion, peeling of the ILM during ERM removal is not necessary in terms of postoperative outcome. In the present study, patients in both treatment groups (ERM only group, ERM/ILM peel group) achieved a similar postoperative decrease in CMT and improvement in BCVA. However, although not statistically significant, patients in the ERM only group showed only a tendency to have more persistent cystoid macular edema postoperatively (incidence of pCME in ERM only peel group was 22.2% vs. 12.9% in the ERM/ILM peel group; p = 0.23). The use of intraoperative triamcinolone appears to be associated with lower occurrence of persistent CME. Not one single patient had to be re-operated due to re-occurrence of ERM. However, because of the relatively short follow-up time, we cannot completely exclude re-occurrence of ERM over the long term. We therefore recommend assessing the presence of cortical vitreous on the ILM after having first removed the ERM. If there is residual vitreous, peeling of the ILM is indicated to prevent re-proliferation of ERM. Surgeons must also consider whether the ILM is intact or damaged after ERM removal. If the ILM is not damaged, the surgeon may decide to leave it; if it is damaged or responsible for retinal striae, removal may offer more complete relief of traction. The surgeon should avoid ILM removal in cases of retinal thinning in patients with circulatory or metabolic disorders [4, 18, 19, 20].
References
Miller B. Epiretinal macular membranes: pathogenesis and treatment. Dev Ophthalmol. 1997;29:61–3.
Guidry C. The role of Müller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75–6.
Geerts L, Pertile G, Sompel van de W. Vitrectomy for epiretinal membranes: visual outcome and prognostic criteria. Bull Soc Belg Ophtalmol. 2004;293:7–15.
Kwok AKH, Lai Tyy, Yuen KSC. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Exp Ophthalmol. 2005;33(4):379–85.
Bovey Eh, Uffer Achache F. Surgery for epimacular membrane: impact of retinal internal limiting membrane removal on functional outcome. Retina. 2004;24(5):728–35.
Hasegawa T, Emi K, Ikeda T. Long-term prognosis of internal limiting membrane peeling for idiopathic epiretinal membrane. Nippon Ganka Gakkai zasshi. 2004;108(3):150–56.
Kenawy K, Wong D, Stappler T. Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology. 2010;117(2):320–323.e1.
Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–67.
Abo El, Enin M, El-Toukhy H, Swelam A. Non-foveal macular holes after PPV for macular pucker. Middle East Afr J Ophthalmol. 2010;17(3):254.
Lee JW, Kim IT. Outcomes of idiopathic macular epiretinal membrane removal with and without internal limiting membrane peeling: a comparative study. Jpn J Ophthalmol. 2010;54(2):129–34.
Tadayoni R, Paques M, Massin P. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001;108(12):2279–2283.
Baba T, Yamamoto S, Kimoto R. Reduction of thickness of ganglion cell complex after internal limiting membrane peeling during vitrectomy for idiopathic macular hole. Eye. 2012;26(9):1173–80.
Shimada H, Nakashizuka H, Hattori T. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009;116(7):1370–76.
Oh HN, Lee JE, Kim KW. Clinical outcomes of double staining and additional ILM peeling during ERM surgery. Korean J Ophthalmol. 2013;27(4):256–60.
Morris R, Kuhn F, Witherspoon CD. Hemorrhagic macular cysts in Terson’s syndrome and its implications for macular surgery. Dev Ophthalmol. 1997;29:44–54.
Kuhn F, Morris R, Witherspoon CD. Terson syndrome: results of vitrectomy and the significance of vitreous hemorrhage in patients with subarachnoid haemorrhage. Ophthalmology. 1998;105(3):472–77.
Kuhn F. Point: to peel or not to peel, that is the question. Ophthalmology. 2002;109(1):9–11.
Gibran SK. Flemming B, Stappler T. Peel and peel again. Br J Ophthalmol. 2008;92(3):373–77.
Kwok AKH, Lai TTY, Li WWY. Indocyanine green-assisted internal limiting membrane removal in epiretinal membrane surgery: a clinical and histologic study. Am J Ophthalmol. 2004;138(2):194–99.
Kwok AKH, Lai TTY, Li WWY, Yew DTW et al. Trypan blue- and indocyanine green-assisted epiretinal membrane surgery: clinical and histopathological studies. Eye. 2004;18(9):882–88.
Sivalingam A, Eagle RC Jr, Duker JS. Visual prognosis correlated with the presence of internal-limiting membrane in histopathologic specimens obtained from epiretinal membrane surgery. Ophthalmology. 1990;97(11):1549–52.
Spaide RF. "Dissociated optic nerve fiber layer appearance" after internal limiting membrane removal is inner retinal dimpling. Retina. 2012;32(9):1719–26.
Acknowledgements
We thank Dr. sc. nat. Sabine Güsewell for her assistance in the statistical analysis.
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure
Josef Guber, Ioana Pereni, Hendrik PN Scholl, Ivo Guber and Richard Haynes have nothing to declare.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the institutional and/or national research committee (NHS Health Research Authority Bristol) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7970864.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Guber, J., Pereni, I., Scholl, H.P.N. et al. Outcomes after Epiretinal Membrane Surgery with or Without Internal Limiting Membrane Peeling. Ophthalmol Ther 8, 297–303 (2019). https://doi.org/10.1007/s40123-019-0185-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-019-0185-7