Abstract
Background
Epiretinal membranes (ERMs) have been reported after pars plana vitrectomy (PPV) for rhegmatogenous retinal detachment (RRD). Peeling of the internal limiting membrane (ILM) can prevent post-PPV ERM formation but has a potential negative impact on macular structure and function.
Purpose
To investigate the anatomical and functional outcomes of ILM peeling during PPV for primary RRD.
Methods
This was a prospective nonrandomized study that included 60 eyes of 60 patients with a primary macula-off RRD and less than grade C proliferative vitreoretinopathy (PVR). Eyes were allocated into 2 groups; Group A underwent PPV without ILM peeling and Group B had ILM peeling. At postoperative month 6, all patients underwent retinal imaging using spectral domain optical coherence tomography (OCT) and OCT angiography and macular function was assessed using multifocal electroretinogram (mfERG). Baseline characteristics and postoperative anatomical and visual outcomes were recorded and statistically analyzed.
Results
We enrolled 30 eyes of 30 patients in each group. In Group A, mean age was 44.6 years, while the mean age of Group B patients was 49.9 years. Postoperative LogMAR visual acuity was significantly better in Group A than in Group B (p < 0.001). ERMs were demonstrated on OCT in 13.3% of Group A and none of Group B patients (p = 0.04). Retinal dimples were found in 53.3% of Group B and none of Group A eyes (p < 0.001). OCTA showed a greater vessel density of the superficial capillary plexus (SCP) in Group A compared to Group B eyes (p = 0.046), while no difference was found regarding deep capillary vessel density (p = 0.7). Mean amplitude of mfERG P1 wave was significantly higher in Group A eyes than in Group B (p = 0.002). Both the SCP vessel density and P1 amplitude were positively correlated with visual acuity (p < 0.001).
Conclusion
This study suggests that ILM peeling prevents ERM development in eyes undergoing PPV for uncomplicated macula-off RRD, but potential damage to macular structure and function were found.
Trial registration Retrospectively registered on 09/24/2019 on ClinicalTrials.gov with an ID of NCT04139811.
Similar content being viewed by others
Background
Internal limiting membrane (ILM) peeling has become an integral step during pars plana vitrectomy (PPV) for managing different macular pathologies, such as macular holes, vitreomacular traction (VMT) syndrome and epiretinal membranes (ERM) [1]. Removal of the ILM has been shown to decrease the recurrence rate of idiopathic ERMs after their surgical removal [2].
ERMs may develop after successful vitrectomy for repair of rhegmatogenous retinal detachment (RRD) with an incidence that varies from 6 to 48% according to published literature [3]. Risk factors for post-PPV ERM development include multiple, large or posterior retinal breaks, as well as a longer duration of macular detachment [4, 5].
ERM development after RRD repair is mostly attributed to migration of retinal pigment epithelial (RPE) cells through retinal breaks and their proliferation on the macular surface. ILM peeling in the setting of primary PPV would remove the scaffold required for these proliferating cells [4]. Several studies evaluated the role of ILM peeling in preventing post-PPV ERM formation and its impact on macular microstructure and function [6,7,8,9,10,11,12,13,14]. The purpose of this study was to assess the anatomical and functional outcomes after ILM peeling during PPV for primary macula-off RRD utilizing optical coherence tomography (OCT), OCT angiography (OCTA) and multifocal electroretinogram (mfERG).
Methods
This was a prospective comparative nonrandomized study. Patients were recruited and surgeries performed at the Department of Ophthalmology, Minia University, Minia, Egypt during the period from March 2017 to September 2019. A written informed consent was obtained from all study participants after thorough explanation of the risks and benefits of surgery and nature of the study. The study was approved by local Ethical Committee of Faculty of Medicine, Minia University and adhered to the tenets of the Declaration of Helsinki.
Patients who were older than 18 years and presented with a primary macula-off RRD with less than grade C proliferative vitreoretinopathy (PVR) were eligible for inclusion in the study. Eyes were excluded if they had prior vitreoretinal surgery or intravitreal injections, combined rhegmatogenous-tractional RD, other macular pathologies, history of glaucoma or corneal opacities that might hinder acquisition of good quality OCT and OCTA images.
Eyes were allocated into 2 groups; Group A underwent PPV without ILM peeling and Group B had ILM peeling. All eyes were operated within 1 week of presentation to avoid progression to advanced PVR. Eyes that had significant cataract underwent phacoemulsification and primary intraocular lens implantation prior to vitrectomy. All PPV surgeries were performed by a single experienced surgeon (MFA). A standard 3-port 23-gauge PPV, using valved one-step transconjunctival trocar and cannula system (DORC International, Zuidland, The Netherlands), was performed for all eyes and perfluorocarbon liquid (PFCL) was used to flatten the retina followed by peripheral vitreous shaving, Endolaser treatment around the causative break(s) and 360° endolaser applied to the periphery. Silicone 1000 cSt was used as a tamponade in all eyes. In Group B eyes, Brilliant Blue G dye 0.025% (ILM-BLUE; DORC International, Zuidland, The Netherlands) was instilled over the detached macula in a fluid-filled eye and left for 30 s followed by PFCL injection. An ILM edge was created at the lower temporal arcade using a diamond-dusted Tano scraper and peeling was completed using an ILM peeling forceps (DORC International). ILM peeling was extended to the major arcades.
Patients were seen at 1 day, 1 week, 1 month and 3 months postoperatively. Silicone oil removal was performed at the 3rd postoperative month, combined with phacoemulsification with intraocular lens (IOL) implantation for all phakic eyes.
Spectral domain (SD) OCT and OCTA imaging was performed for all eyes in the absence of silicone oil at the 6th postoperative month after the original surgery. OCT and OCTA imaging were done using Avanti RTVue-XR platform (Optovue Inc., Fremont, CA) which uses a split-spectrum amplitude-decorrelation angiography (SSADA) algorithm. Two consecutive B-scans are obtained at each location before moving to another one. Each OCTA volume contains 304 × 304 A-scans. In order to minimize motion artifacts, 2 orthogonal OCTA volumes are obtained. The Angio Retina protocol was used for OCTA image acquisition, with a scan area of 6 × 6 mm. Automated segmentation was used to define the different vascular plexuses. OCT parameters evaluated were the central macular thickness (CMT), presence or absence of ERM and retinal dimples. OCTA images were evaluated for vessel density (VD) of both the superficial capillary plexus (SCP) and deep capillary plexus (DCP). The vessel area density (VD) was calculated using the Vessel Density Map of the AngioAnalytics software (RTVue-XR version: 2017.1.0.151, Optovue) as the percentage area occupied by vessels relative to the total scan area at the level of the SCP and DCP. All OCT and OCTA images were evaluated by 2 experienced observers.
Multifocal electroretinogram (mfERG) was recorded for all eyes at the 6th postoperative month using RetiPort System (Roland Consult, Brandenburg, Germany). The test was performed according to the standards of the International Society of Clinical Electrophysiology of Vision (ISCEV) [15]. The P1 amplitude was evaluated.
In mfERG recording procedure, an active HK-loop electrode (Roland Consult) was applied to the lower conjunctival fornix after 10 min of light adaptation and pupil dilatation with tropicamide 1% eye drops. A ground electrode was applied to the forehead and a reference electrode was connected to the ipsilateral temple. The standard mfERG visual stimulus was used, which consists from 61 hexagons covering the central 25–30° of the visual field, and presented on a 20-inch monitor at a distance of 33 cm. For each hexagon, the amplitude of P1 (defined as the difference between the trough of N1; first negative wave, and the peak of P1; second positive wave) was calculated.
Statistical analyses
The collected data were coded, tabulated and statistically analyzed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY). Parametric quantitative data were expressed as mean ± standard deviation, non-parametric quantitative data as median and interquartile range (IQR), and categorical data as numbers and percentages. Distribution of data was done using Kolmogorov–Smirnov test. Comparison between both study groups was done using Independent Samples T test for parametric quantitative data and Mann–Whitney test for non-parametric quantitative data. Analyses were done for qualitative data using Chi square test (if < 20% of cells had an expected count < 5) and Fisher’s exact test (if > 20% of cells had an expected count < 5). p value < 0.05 was considered significant. Correlation analysis between variables was done applying Spearman’s ranked correlation test for non-parametric data.
Results
Baseline characteristics
We enrolled 30 eyes of 30 patients in each group. In Group A, mean age was 44.6 years and 24 patients were males (80%). The mean age of Group B participants was 49.9 years and males constituted 76.7% (23 patients). Both groups were balanced regarding their age, sex, lens status, extent of RD and number of breaks (Table 1).
Visual outcomes
All eyes in both groups had macula-off RRD, defined as complete involvement of the fovea by subretinal fluid as detected clinically by fundus biomicroscopy, with a mean preoperative LogMAR BCVA of 1.9 ± 0.5 in group A and 1.9 ± 0.3 in group B. Postoperative LogMAR BCVA was significantly better in Group A (non-ILM peeling) than in Group B at the 6th postoperative month (p < 0.001). Mean LogMAR BCVA was 0.6 ± 0.2 in Group A (range 0.3–0.9) and 0.9 ± 0.15 in Group B (range 0.6–1.0).
OCT parameters
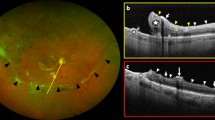
ERMs were demonstrated on SD OCT in 4 cases (13.3%) of Group A and none of Group B patients at 6 months (p = 0.04). Only 3 eyes in Group A had significant cataract and underwent phacovitrectomy, and none of them developed an ERM. No eyes in Group B had significant cataract that required lens removal before PPV. Retinal dimples were evident in 16 Group B patients (53.3%) and none of Group A patients (p < 0.001). No significant difference was found between both groups regarding postoperative CMT (262.1 ± 54.4 μm in Group A vs 262.3 ± 38.7 μm in Group B, p = 0.99) (Fig. 1).
a B-scan SD-OCT of the macula of a Group A patient showing a thick epiretinal membrane (ERM) causing macular pucker, thickening and cystoid degeneration after pars plana vitrectomy. b B-scan SD-OCT of the macula of a Group B patient showing dimples on the inner retinal surface after pars plana vitrectomy and peeling of the internal limiting membrane (ILM)
Octa parameters
Mean vessel density of the SCP was 44.8 ± 6.4% in Group A and 41.7 ± 5.5% in Group B, a difference that barely reached statistical significance (p = 0.046). Moreover, greater SCP vessel density was significantly correlated with better visual acuity (r = − 0.83, p < 0.001). No significant difference in VD of the DCP was found between both groups (42.3 ± 5.8% in Group A vs 41.7 ± 5.4% in Group B, p = 0.7) (Fig. 2).
Top row represents a Group A eye. a An optical coherence tomography angiography (OCTA) macula 6 × 6 mm scan showing the slab representing the superficial capillary plexus (SCP). b En-face OCT image that corresponds to the OCTA slab in A. c The color-coded flow density map of the superficial vessel density (warmer colors represent greater flow density). Bottom row represents a Group B eye. d An OCTA macula 6 × 6 mm scan showing the slab representing the superficial capillary plexus (SCP). e En-face OCT image that corresponds to the OCTA slab in D showing retinal dimples at the level of the nerve fiber layer. f The color-coded flow density map of the superficial vessel density
Multifocal ERG
Mean amplitude of P1 wave was significantly higher in Group A eyes (40.1 ± 9.8 nV/deg2) compared to Group B (30.9 ± 11.8 nV/deg2, p = 0.002). Furthermore, the P1 amplitude was strongly correlated to BCVA (r = -0.9, p < 0.001) (Fig. 3). Table 2 summarizes the postoperative outcomes for both groups.
a Multifocal electroretinogram (mfERG) of a Group A eye. Left. Amplitude of P1 in nV/deg2 in topographic display around the fovea. Right. Three-dimensional topography of P1 amplitude (nV/deg2). b Multifocal electroretinogram (mfERG) of a Group B eye. Left. Amplitude of P1 in nV/deg2 in topographic display around the fovea. Right. Three-dimensional topography of P1 amplitude (nV/deg2)
Discussion
ILM peeling has been shown by several investigators to prevent ERM formation after vitrectomy for repair of RRD of different degrees of complexity [6,7,8,9,10,11,12,13,14]. We included only eyes with primary RRD and no more than grade B PVR, because we sought to assess the benefit of ILM peeling as a routine step during repair of uncomplicated RRD. Advanced PVR could be associated with higher risk for postoperative ERM development and inclusion of these eyes might have confounded our anatomical results [13, 16]. We also excluded eyes with fovea-sparing RRDs to avoid any potential complications of performing ILM peeling on a non-diseased macula.
A recent meta-analysis of studies comparing ILM peeling versus no peeling during PPV for RRD found the incidence of postoperative macular ERM formation to be significantly lower in patients who had ILM peeling, but this bared no significant effect on postoperative visual outcomes [3].
In agreement with previous studies, eyes that had ILM peeling in our study developed no ERMs at postoperative month 6, while an ERM was demonstrated on SD OCT in 4 eyes (13.3%) that had no ILM peeling (p = 0.04). Indeed, the incidence of postoperative ERM development in eyes that underwent ILM peeling during PPV for RRD ranged from 0 to 9% in several publications [6, 7, 9, 13, 14]. This is to be expected as ILM peeling ensures complete removal of the posterior hyaloid cortex and deprives RPE cells from the support they need to proliferate on the macular surface [17, 18].
We performed a thorough vitreous base shaving in all eyes and peripheral 360 degrees endolaser was done in order to avoid missing any minute holes, which might not have been visualized during surgery, which may lead to RD recurrence. Although peripheral 360 degrees endolaser was performed in all eyes in both groups, none of the eyes in group B developed an ERM while ERMs were demonstrated in 4 eyes (13.3%) of Group A, suggesting that peripheral endolaser did not appear to increase incidence of ERMs.
We used silicone 1000 cSt in all eyes due to the frequent shortages of expansile gases in our locality, while silicone oil is always available, to ensure that all eyes received the same tamponade and eliminate any confounding variable that might affect postoperative macular anatomy and function.
Several studies have shown that ILM peeling may cause microstructural mechanical damage to the retina in the form of retinal dimples, dissociated nerve fiber layer (DONFL) and focal retinal thinning [19]. This trauma has been attributed to the impact on the macular surface of instruments used to peel the ILM [20, 21].
Retinal dimples developed postoperatively in 16 Group B eyes (53.3%) and none of Group A eyes (p < 0.001). Recent studies demonstrated inner retinal dimples in 100% of eyes that underwent PPV with ILM peeling for RRD repair [9, 22]. The incidence of retinal dimples was reported to be 68% after ILM removal for full-thickness and lamellar macular holes [23]. Retinal dimples are hypothesized to be the result of diffuse damage to Müller cell end-feet. The impact of these changes on macular function is still unclear [24, 25]. Concordant with the results of Eissa and associates [9], we found no significant difference in postoperative CMT between the 2 groups.
To the best of our knowledge, this is the first study to use OCTA for evaluating the effect of ILM peeling on macular microvasculature after PPV for RRD. OCTA is a recent addition to the expanding arsenal of retinal imaging modalities, and has provided novel insights into the pathogenesis of different macular diseases [26]. Mastropasqua and colleagues used OCTA to study SCP changes after ILM peeling for idiopathic ERMs. They noted a reduction in the SCP vessel density which was evident in areas of microstructural alterations on SD OCT such as swelling of the arcuate nerve fiber layer. They hypothesized that these changes resulted from the direct surgical trauma to the inner retina which contains the SCP [27]. Similarly we found a marginally significant reduction in SCP vessel density among Group B eyes compared to Group A (p = 0.046). However, no significant difference existed between both groups regarding the DCP vessel density, most likely because of the DCP was not directly impacted by surgical manipulation.
Group A eyes had significantly better BCVA than Group B eyes at final follow-up (p < 0.001). Furthermore, postoperative vision significantly and positively correlated with SCP vessel density and mfERG P1 wave amplitude (p < 0.001). This comes in agreement with Eissa et al. [9] Other studies have reported a trend towards better visual acuity in non-ILM peeling eyes compared to eyes that had ILM peeling, though this difference did not reach statistical significance [8, 10, 14]. Interestingly, Aras et al. demonstrated similar visual outcomes between eyes that had ILM peeling during PPV for complex RRDs compared to those with no ILM peeling [12]. This might indicate that the magnitude of effect that ILM peeling had on visual function was less meaningful in eyes with complicated pathology and, hence, ILM peeling could be more justified in such context.
Multifocal ERG is an objective method to evaluate macular function. The P1 peak is thought to originate from the inner retinal cells, namely bipolar and Muller cells and this is why we chose to use P1 amplitude in our analysis [15, 28]. We found a significant reduction in P1 amplitude in Group B eyes compared to Group A and this reduction correlated with lower BCVA and SCP vessel density (p < 0.001).
Other studies utilized mfERG to assess macular function after ILM peeling for idiopathic ERMs. Lim et al. found nonsignificant reduction in postoperative P1 amplitude compared to preoperative values. This reduction did not recover at 1 year postoperatively, despite visual and anatomical improvement [29]. Another study found slight reduction in mfERG amplitudes that was accompanied by an asymptomatic decrease in visual field sensitivity in a small group of patients after ILM peeling for idiopathic macular pucker [30].
Eissa et al. found significant reduction in mean and foveal retinal sensitivity as measured by microperimetry in the ILM versus none-ILM peeling group. Both microperimetry measures correlated with final BCVA [9]. All these findings point the functional deficit that can be caused by ILM peeling even in asymptomatic patients.
Conclusions
In conclusion, this study suggests that ILM peeling prevents ERM development in eyes undergoing PPV for uncomplicated macula-off RRD, but potential damage might occur to macular structure and function. Limitations of our study include the small sample size, lack of randomization and relatively short follow-up period. Among the strengths of our study are the use of strict selection criteria and utilization of recent modalities to assess macular structure and function such as OCTA and mfERG. It is our belief that ILM peeling should be reserved for more complex cases of RD with advanced PVR rather than be incorporated as a routine step during primary RD repair.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCVA:
-
Best-corrected visual acuity
- CMT:
-
Central macular thickness
- DCP:
-
Deep capillary plexus
- DONFL:
-
Dissociated optic nerve fiber layer
- ERM:
-
Epiretinal membrane
- ILM:
-
Internal limiting membrane
- IOL:
-
Intraocular lens
- IQR:
-
Interquartile range
- ISCEV:
-
International Society of Clinical Electrophysiology of Vision
- LogMAR:
-
Logarithm of minimum angle resolvable
- mfERG:
-
Multifocal electroretinogram
- OCTA:
-
Optical coherence tomography angiography
- PFCL:
-
Perfluorocarbon liquid
- PPV:
-
Pars plana vitrectomy
- PVR:
-
Proliferative vitreoretinopathy
- RPE:
-
Retinal pigment epithelium
- RRD:
-
Rhegmatogenous retinal detachment
- SCP:
-
Superficial capillary plexus
- SD OCT:
-
Spectral domain optical coherence tomography
- SSADA:
-
Split-spectrum amplitude-decorrelation angiography
- VMT:
-
Vitreomacular traction
References
Abdelkader E, Lois N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv Ophthalmol. 2008;53(4):368–96.
Sandali O, El Sanharawi M, Basli E, Bonnel S, Lecuen N, Barale PO, Borderie V, Laroche L, Monin C. Epiretinal membrane recurrence: incidence, characteristics, evolution, and preventive and risk factors. Retina. 2013;33(10):2032–8.
Fallico M, Russo A, Longo A, Pulvirenti A, Avitabile T, Bonfiglio V, Castellino N, Cennamo G, Reibaldi M. Internal limiting membrane peeling versus no peeling during primary vitrectomy for rhegmatogenous retinal detachment: A systematic review and meta-analysis. PLoS One. 2018;13:7.
Katira RC, Zamani M, Berinstein DM, Garfinkel RA. Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina. 2008;28(5):744–8.
Martinez-Castillo V, Boixadera A, Distefano L, Zapata M, Garcia-Arumi J. Epiretinal membrane after pars plana vitrectomy for primary pseudophakic or aphakic rhegmatogenous retinal detachment: incidence and outcomes. Retina. 2012;32(7):1350–5.
Nam KY, Kim JY. Effect of internal limiting membrane peeling on the development of epiretinal membrane after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2015;35(5):880–5.
Forlini M, Date P, Ferrari LM, Lorusso M, Lecce G, Verdina T, Neri G, Benatti C, Rossini P, Bratu A, et al. Comparative analysis of retinal reattachment surgery with or without internal limiting membrane peeling to prevent postoperative macular pucker. Retina. 2018;38(9):1770–6.
Akiyama K, Fujinami K, Watanabe K, Tsunoda K, Noda T. Internal limiting membrane peeling to prevent post-vitrectomy epiretinal membrane development in retinal detachment. Am J Ophthalmol. 2016;171:1–10.
Eissa M, Abdelhakim M, Macky TA, Khafagy MM, Mortada HA. Functional and structural outcomes of ILM peeling in uncomplicated macula-off RRD using microperimetry & en-face OCT. Graefes Arch Clin Exp Ophthalmol. 2018;256(2):249–57.
Rao RC, Blinder KJ, Smith BT, Shah GK. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology. 2013;120(5):1102–3.
Garweg JG, Deiss M, Pfister IB, Gerhardt C. Impact of inner limiting membrane peeling on visual recovery after vitrectomy for primary rhegmatogenous retinal detachment involving the fovea. Retina. 2019;39(5):853–9.
Aras C, Arici C, Akar S, Muftuoglu G, Yolar M, Arvas S, Baserer T, Koyluoglu N. Peeling of internal limiting membrane during vitrectomy for complicated retinal detachment prevents epimacular membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):619–23.
Foveau P, Leroy B, Berrod JP, Conart JB. Internal limiting membrane peeling in macula-off retinal detachment complicated by grade b proliferative vitreoretinopathy. Am J Ophthalmol. 2018;191:1–6.
Blanco-Teijeiro MJ, Bande Rodriguez M, Mansilla Cunarro R, Paniagua Fernandez L, Ruiz-Oliva Ruiz F, Pineiro Ces A. Effects of internal limiting membrane peeling during vitrectomy for macula-off primary rhegmatogenous retinal detachment. Eur J Ophthalmol. 2018;28(6):706–13.
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM. ISCEV standard for clinical multifocal electroretinography (mfERG). Documenta ophthalmologica Adv Ophthalmol. 2012;124(1):1–13.
Kiss CG, Richter-Muksch S, Sacu S, Benesch T, Velikay-Parel M. Anatomy and function of the macula after surgery for retinal detachment complicated by proliferative vitreoretinopathy. Am J Ophthalmol. 2007;144(6):872–7.
Hisatomi T, Enaida H, Sakamoto T, Kanemaru T, Kagimoto T, Yamanaka I, Ueno A, Nakamura T, Hata Y, Ishibashi T. Cellular migration associated with macular hole: a new method for comprehensive bird’s-eye analysis of the internal limiting membrane. Arch Ophthalmol. 2006;124(7):1005–11.
Hisatomi T, Enaida H, Sakamoto T, Kagimoto T, Ueno A, Nakamura T, Hata Y, Ishibashi T. A new method for comprehensive bird’s-eye analysis of the surgically excised internal limiting membrane. Am J Ophthalmol. 2005;139(6):1121–2.
Hisatomi T, Tachibana T, Notomi S, Koyanagi Y, Murakami Y, Takeda A, Ikeda Y, Yoshida S, Enaida H, Murata T, et al. Internal limiting membrane peeling-dependent retinal structural changes after vitrectomy in rhegmatogenous retinal detachment. Retina. 2018;38(3):471–9.
Deltour JB, Grimbert P, Masse H, Lebreton O, Weber M. Detrimental effects of active internal limiting membrane peeling during epiretinal membrane surgery: microperimetric analysis. Retina. 2017;37(3):544–52.
Steel DH, Dinah C, Habib M, White K. ILM peeling technique influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):691–8.
Fukukita H, Ito Y, Iwase T, Kaneko H, Yasuda S, Kataoka K, Terasaki H. Inner macular changes after vitrectomy with internal limiting membrane peeling for rhegmatogenous retinal detachment: similarity with alport syndrome. Retina. 2018;39(12):2332–40.
Liu J, Chen Y, Wang S, Zhang X, Zhao P. Evaluating inner retinal dimples after inner limiting membrane removal using multimodal imaging of optical coherence tomography. BMC Ophthalmol. 2018;18(1):155.
Alkabes M, Salinas C, Vitale L, Bures-Jelstrup A, Nucci P, Mateo C. En face optical coherence tomography of inner retinal defects after internal limiting membrane peeling for idiopathic macular hole. Invest Ophthalmol Vis Sci. 2011;52(11):8349–55.
Rispoli M, Le Rouic JF, Lesnoni G, Colecchio L, Catalano S, Lumbroso B. Retinal surface en face optical coherence tomography: a new imaging approach in epiretinal membrane surgery. Retina. 2012;32(10):2070–6.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Mastropasqua L, Borrelli E, Carpineto P, Toto L, Di Antonio L, Mattei PA, Mastropasqua R. Microvascular changes after vitrectomy with internal limiting membrane peeling: an optical coherence tomography angiography study. Int Ophthalmol. 2018;38(4):1465–72.
Hood DC, Greenstein V, Frishman L, Holopigian K, Viswanathan S, Seiple W, Ahmed J, Robson JG. Identifying inner retinal contributions to the human multifocal ERG. Vision Res. 1999;39(13):2285–91.
Lim JW, Cho JH, Kim HK. Assessment of macular function by multifocal electroretinography following epiretinal membrane surgery with internal limiting membrane peeling. Clin Ophthalmol. 2010;4:689–94.
Tari SR, Vidne-Hay O, Greenstein VC, Barile GR, Hood DC, Chang S. Functional and structural measurements for the assessment of internal limiting membrane peeling in idiopathic macular pucker. Retina. 2007;27(5):567–72.
Acknowledgements
Not applicable.
Funding
No funding sources.
Author information
Authors and Affiliations
Contributions
MEA contributed in acquisition of data and writing the manuscript. HMM contributed by performing silicone removal surgeries, analysis and interpretation of data. ASA contributed in acquisition of data and writing the manuscript. KMM contributed by analysis and interpretation of data, and critically reviewing the manuscript. MFA contributed by performing vitrectomy surgeries, analysis and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by local Ethical Committee of Faculty of Medicine, Minia University with a reference number of 66-7/2018.
Consent for publication
No consent for publication was obtained from the patients as all the images used are entirely unidentifiable and there are no personal data included in the manuscript that can be traced to an individual patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdullah, M.E., Moharram, H.E.M., Abdelhalim, A.S. et al. Evaluation of primary internal limiting membrane peeling in cases with rhegmatogenous retinal detachment. Int J Retin Vitr 6, 8 (2020). https://doi.org/10.1186/s40942-020-00213-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40942-020-00213-4