Abstract
Introduction
The aim of this study was to evaluate retinal and choriocapillaris (CC) vessel density, using optical coherence tomography angiography (OCTA), in the macular region at baseline and after three once-monthly intravitreal injections of bevacizumab (loading phase) in patients affected by exudative age-related macular degeneration (AMD).
Methods
A total 38 eyes of 38 patients with exudative AMD (16 women, 22 men; mean age 72.47 ± 7.46 years) were included in this study. We evaluated vessel density in different macular areas (whole image, fovea and parafovea) of the superficial capillary plexus (SCP), deep capillary plexus (DCP) and CC. In addition, central macular thickness (CMT) and choroidal thickness in the foveal region and 500 µm in the nasal and temporal directions were analyzed by enhanced depth image-OCT.
Results
There were no significant differences in vessel density in the SCP, DCP and CC after the loading phase compared to baseline (p > 0.05). In contrast, CMT (p = 0.039; 320.63 ± 175.05 vs. 372.47 ± 167.05 µm) and subfoveal choroidal thickness (SFCT) (p < 0.001; 189.21 ± 37.66 µm vs 170.37 ± 37.96 µm) significantly decreased after the loading phase compared to baseline. No significant correlations were found between SFCT and CC vessel density in the fovea, and between CMT and SCP and DCP (p > 0.05) at baseline and after the loading phase. Also, best corrected visual acuity did not correlate with CMT, SCP and DCP at baseline and after treatment (p > 0.05).
Conclusion
Macular and choroidal thickness did not correlate with vessel density, but probably correlated to vascular exudation. The anti-VEGF treatment, acting on vascular hyperpermeability, determines the reduction of the retinal and choroidal exudation, resulting in a decrease of their thicknesses. Consequently, these parameters could reflect the effectiveness of the anti-VEGF injections for treating exudative AMD respect to OCTA parameters.
Trial Registration
ClinicalTrials.gov indentifier, NTC04138420.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Intravitreal injections of anti-vascular endothelial growth factor (VEGF) represent the main treatment of exudative age-related macular degeneration (AMD). |
This anti-VEGF therapy could cause changes in retinal and choroidal thickness and in their vascular networks. |
The aim of this study was to evaluate (1) retinal and choriocapillaris vessel density in the macular region using optical coherence tomography angiography (OCTA) and (2) central macular thickness (CMT) and subfoveal choroidal thickness (SFCT) using structural OCT, at baseline and after three once-monthly intravitreal injections of bevacizumab (loading phase) in these patients. |
What was learned from the study? |
No significant differences in vessel density were present after the loading phase compared to baseline, while the CMT and SFCT did significantly decrease after the loading phase. |
The thicknesses of the macula and choroid were not correlated to vessel density, but they could be correlated to vascular exudation. |
The anti-VEGF treatment (bevacizumab), acting on vascular hyperpermeability, determines the level of reduction of retinal and choroidal exudation, resulting in a decrease of their thicknesses; consequently, these parameters could reflect the effectiveness of the anti-VEGF injections for treating exudative AMD with respect to OCTA parameters. |
Introduction
Exudative age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in developed countries [1].
In recent years, intravitreal injections of vascular endothelial growth factor (VEGF) antagonists have become the first-line treatment for exudative AMD. Anti-VEGF therapy has enabled exudation related to choroidal neovascularization (CNV) to be resolved [2, 3]. The effectiveness of this treatment had been evaluated by optical coherence tomography (OCT), a useful diagnostic tool that allows a detailed evaluation of choroidal and macular thickness [4, 5]; the spectral domain (SD)-OCT with enhanced depth image (EDI) technique in particular provides very accurate assessments of choroidal morphology [6].
Previous studies have reported that one effect of anti-VEGF treatment of exudative AMD is a significant reduction in the thicknesses of the choroid and macula [7,8,9,10,11,12,13].
The introduction of optical coherence tomography angiography (OCTA), a novel and non-invasive diagnostic technique, into clinical practice has enabled clinicians to analyze vessel density of the retina and choriocapillaris (CC) quantitatively and in great detail [14, 15]. There have been several studies using OCTA that focused on CNV contraction after anti-VEGF treatment; to date, however, there are no studies on the changes in retinal and choroidal vascular density at baseline and after anti-VEGF treatment or on the correlations between structural OCT and OCTA parameters in exudative AMD [16,17,18].
The aim of this study was to determine, using OCTA and EDI-OCT, retinal and choroidal vascular features under the effects of bevacizumab intravitreal injections in patients with exudative AMD.
Methods
In this prospective study, we enrolled a total of 38 consecutive eyes of 38 patients (16 women, 22 men; mean age 72.47 ± 7.46 years) affected by exudative AMD who consecutively presented to the Eye Clinic of the University of Naples “Federico II” from January to October 2017. Criteria for inclusion were age > 50 years and diagnosis of treatment-naïve exudative AMD due to the presence of type 1 or type 2 CNV. Exclusion criteria included CNV secondary to causes other than AMD; idiopathic polypoidal choroidal vasculopathy; previous treatments for CNV prior to bevacizumab (such as laser photocoagulation, photodynamic therapy, intravitreal injections of other anti-VEGF); geographic atrophy; subretinal fibrosis; vitreoretinal disease; retinal vascular disease; myopia > 6 diopters (D); history of ocular surgery; and significant lens opacity. We also excluded images with visible eye motion, blinking artifacts and low-quality images obtained with OCTA.
Each subject underwent evaluation of best-corrected visual acuity (BCVA) according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) [19], intraocular pressure (IOP) measurement, slit-lamp biomicroscopy, fundus examination with a + 90-D lens, fluorescein angiography, indocyanine green angiography, structural SD-OCT and OCTA (RTVue XR Avanti, Optovue, Inc., Freemont, CA, USA). Choroidal thickness (CT) in the subfoveal region (SFCT) and 500 µm in the nasal and temporal directions was measured using the Spectralis Heidelberg diagnostic imaging platform with EDI mode (Heidelberg Engineering, Heidelberg, Germany).
All patients received three once-monthly intravitreal injections of bevacizumab (1.25 mg/0.05 mL) (loading phase) with a 30-gauge needle. The injection was made through the pars plana under aseptic conditions. At baseline and 1 month after the third bevacizumab monthly injection all patients underwent assessment of BCVA and ophthalmic examination, including slit-lamp biomicroscopy, IOP, fundus biomicroscopy, SD-OCT and OCTA.
Two independent observers (GC, DM) carefully reviewed the EDI-OCT and OCTA images, with the aim to measure SFCT and to analyze segmentation of the vascular layers, respectively.
The research protocol was approved by the Institutional Review Board of the University of Naples “Federico II” (PT 1124/14; ClinicalTrials.gov indentifier, NTC04138420). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
EDI-OCT Measurement
The SFCT was measured using the Spectralis OCT device in EDI mode (Heidelberg Engineering).
CT was evaluated in the subfoveal region and 500 µm in the nasal and temporal directions as a manual linear measurement between the outer border of Bruch’s membrane and the most posterior identifiable aspect of the choroidal–scleral interface, which is seen as a hyper-reflective layer in the posterior margin of the choroid in EDI mode [20].
Optical Coherence Tomography Angiography
The OCTA images were obtained using the Optovue Angiovue System (software ReVue version 2014.2.0.93; Optovue Inc., Fremont, CA, USA) which is based on the split-spectrum amplitude de-correlation algorithm. The instrument has an A-scan rate of 70,000 scans per seconds with a tissue axial resolution of 5 µm and a 15-µm beam width. Each B-scan contained 304 A-scans. Two consecutive B-scans were captured at a fixed position before proceeding to the next sampling location. Blood flowing through vessels causes a change in reflectance over time and results in localized areas of flow de-correlation between frames. The spectrum of the light source was split into multiple component parts to decrease the noise present in the image; each part was used to perform the de-correlation step, and the results of all the split spectra were averaged. In any given region of tissue, the projection image can be viewed to obtain an image of the contained blood flow [21]. Cross-sectional registered reflectance intensity images and flow images were summarized and viewed as an en face maximum flow projection from the inner limiting layer to the retinal epithelial pigment. The macular capillary network was visualized in scans centered on the fovea by performing a 6 × 6-mm scan over the macular region. Vessel density was defined as the percentage area occupied by the large vessels and microvasculature in the analyzed region [22]. The OCTA software, in accordance to the ETDRS classification of diabetic retinopathy, applies a grid centered on the fovea to all angiograms; this grid divides the macular region in the foveal and parafoveal areas. For each eye analyzed, the software automatically calculates vessel density in the whole scan area and in all sections of the applied grid in different vascular networks of the retina, namely the superficial capillary plexus (SCP) and deep capillary plexus (DCP) and the CC. Poor-quality images with a signal strength index of < 40 or registered image sets with residual motion artefacts were excluded from the analysis.
Statistical Analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (version 20.0 for Windows; IBM Corp., Armonk, NY, USA). The paired Student’s test was used to evaluate differences in vessel density in the CC and retina, in the SFCT between the baseline and after three once-monthly intravitreal bevacizumab injections. The relationships between SFCT and CC vessel density and between central macular thickness (CMT) and SCP and DCP, respectively, were evaluated by Pearson’s correlation. A p value of < 0.05 was considered to be statistically significant.
Results
A total of 38 eyes of 38 patients (16 women, 22 men; mean age 72.47 ± 7.46 years) were included in this study. No statistically significant difference in BCVA was shown between baseline and after the loading phase.
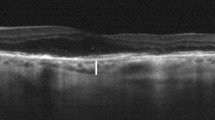
Analysis of the OCT parameters revealed that CMT and CT (in subfoveal region; 500 µm in the nasal and temporal directions; and average CT) were significantly reduced after the loading phase in comparison to baseline (Table 1; Fig. 1).
Optical coherence tomography (OCT) and OCT angiography (OCTA) images. Baseline (top row) versus post-treatment (3 once-monthly intravitreal injections of bevacizumab) follow-up (bottom row) in the right eye of a patient affected by exudative age-related macular degeneration. a, e Structural OCT B-scan showing increased central macular thickness and subfoveal choroidal thickness at baseline (a) compared to post-treatment (e). b–h OCTA images of the superficial capillary plexus, deep capillary plexus and choriocapillaris at baseline (b, c, d, respectively) and after loading phase (f, g, h, respectively). Images show that there are no significative changes in vessel density between baseline and after loading phase
Conversely, there was no statistically significant difference in vessel density in all sectors of the CC between baseline and after the loading phase. Regarding retinal vasculature, the SCP and DCP also showed no significant differences in vessel density in all macular sectors before and after anti-VEGF injections (Table 2).
No significant correlation was found at either time point, i.e. baseline and after the loading phase, between SFCT and CC vessel density in the foveal region, and between MCT and SCP and DCP in the foveal region. In addition, BCVA at both baseline and after treatment did not correlate with the OCT and OCTA parameters (Table 3).
Discussion
In this prospective study, we used OCTA findings to perform a quantitative analysis of retinal and choriocapillaris vessel density in patients with exudative AMD before and after three once-monthly bevacizumab injections. Analyzing the retinal and CC vascular network, we found that vessel density did not differ significantly in patients at baseline and after the loading phase in all macular sectors. In contrast, CMT and SFCT, evaluated by EDI-OCT, significantly decreased after the treatment.
These findings could possibly be explained by the action of the intravitreal anti-VEGF injections in the treatment of exudative AMD, which could involve the macular and choroidal thickness but not the retinal and choriocapillaris vascular blood flow. We hypothesized that the anti-VEGF treatment may influence retinal and choroidal exudation, reducing vascular hyperpermeability and resulting in a significant reduction of CMT and SFCT. Similar findings have been reported in earlier studies [23,24,25,26].
Several studies have shown that VEGF is an important factor in terms of increasing vascular permeability because it modifies the blood–retina barrier, thereby influencing retinal and choroidal exudation. In particular, VEGF downregulates the retinal pigment epithelium tight-junction proteins and the tight junctions between retinal capillary endothelial cells, which in turn leads to a breakdown of the outer and inner blood–retina barriers, respectively [27, 28]. The anti-VEGF that is injected is able to penetrate these barriers and act on the choroidal and retinal vascularizations, reducing their vascular permeability [29, 30].
Our results are in accordance with those of previous studies that used different techniques [31,32,33,34]. Our study reveals the absence of influence of the anti-VEGF injections on the vascular blood flow, with a significant reduction only in vessel diameter of the retina and choroid.
Regarding the functional aspects, in our study BCVA did not improve after the three once-monthly injections of bevacizumab and it also did not show significant correlations with CMT, probably due to changes in the outer retinal layers involved in the exudative alterations, as has also been shown by several earlier studies [35,36,37].
Our study has a number of limitations; these include the relatively small sample size of the groups of subjects and the inability of the EDI-OCT software to automatically measure the choroidal thickness.
Conclusion
In conclusion, we found that OCTA provides detailed evidence on the vascular features of the retina and CC in exudative AMD patients. Unlike OCTA parameters, SFCT and CMT represent reliable parameters that could reflect the effectiveness of the anti-VEGF injections for treating macular edema and choroidal thickening secondary to exudative AMD. Further studies with a larger study group and a longer follow-up are needed to better explain our findings.
Change history
22 December 2021
The license text was incorrectly structured. The article has been corrected.
References
Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1.
CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Heier JS, Brown DM, Chong V. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Regatieri CV, Branchini L, Duker JS. The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42[Suppl]:S56–66.
Fang PP, Domdei N, Herrmann P, et al. Minimal optical coherence tomography B-scan density for reliable detection of intraretinal and subretinal fluid in macular diseases. Retina. 2019;39:150–6.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Branchini L, Regatieri C, Adhi M, et al. Effect of intravitreous antivascular endothelial growth factor therapy on choroidal thickness in neovascular age-related macular degeneration using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131:693–4.
Mazaraki K, Fassnacht-Riedele H, Blum R, Becker M, Michels S. Change in choroidal thickness after intravitreal aflibercept in pretreated and treatmentnaive eyes for neovascular age-related macular degeneration. Br J Ophthalmol. 2016;99:1341–4.
McDonnell EC, Heussen FM, Ruiz-Garcia H, et al. Effect of anti-VEGF treatment on choroidal thickness over time in patients with neovascular age related macular degeneration. Eur J Ophthalmol. 2014;24:897–903.
Framme C, Panagakis G, Birngruber R. Effects on choroidal neovascularization after anti-VEGF upload using intravitreal ranibizumab, as determined by spectral domain-optical coherence tomography. Investig Ophthalmol Vis Sci. 2010;51:1671–6.
Ünlü C, Erdogan G, Gunay BO, Kardes E, Akcay BI, Ergin A. Subfoveal choroidal thickness changes after intravitreal bevacizumab injection for neovascular age-related macular degeneration and diabetic macular edema. Int Ophthalmol. 2017;37:147–58.
Landa G, Amde W, Doshi V, et al. Comparative study of intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) in the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2009;223:370–5.
Spooner K, Hong T, Nair R, et al. Long-term outcomes of switching to aflibercept for treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2019;97:e706–12.
Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35:2196–203.
Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58–67.
Coscas F, Lupidi M, Boulet JF, et al. Optical coherence tomography angiography in exudative age-related macular degeneration: a predictive model for treatment decisions. Br J Ophthalmol. 2018;103:1342–6.
Pilotto E, Frizziero L, Daniele AR, et al. Early OCT angiography changes of type 1 CNV in exudative AMD treated with anti-VEGF. Br J Ophthalmol. 2019;103:67–71.
Abri Aghdam K, Seidensticker F, Pielen A, Framme C, Junker B. The short-term effects of aflibercept on the size of choroidal neovascularization lesion in treatment-resistant neovascular age-related macular degeneration as determined by spectral-domain optical coherence tomography. Lasers Surg Med. 2016;48:668–77.
Kniestedt C, Stamper RL. Visual acuity and its measurement. Ophthalmol Clin N Am. 2003;16:155–70.
Coscas F, Puche N, Coscas G, et al. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Investig Ophthalmol Vis Sci. 2014;55:64–9.
Jia Y, Tan O, Tokayer J, Subhash H, Fujimoto JG, Hornegger J, Huang D, et al. Split spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25.
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the optovue device. Dev Ophthalmol. 2016;56:6–12.
Razavi S, Souied EH, Darvizeh F, Querques G. Assessment of choroidal topographic changes by swept-source optical coherence tomography after intravitreal ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2015;160:1006–13.
Palkovits S, Seidel G, Pertl L, et al. Macular choroidal volume changes after intravitreal Bevacizumab for exudative age-related macular degeneration. Retina. 2017;37:2262–8.
Amarakoon S, Martinez-Ciriano JP, van den Born LI, Baarsma S, Missotten T. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of on-demand therapy every 4 or 8 weeks. Acta Ophthalmol. 2019;97:107–12.
Sizmaz S, Kucukerdonmez C, Kal A, Pinarci EY, Canan H, Yilmaz G. Retinal and choroidal thickness changes after single anti-VEGF injection in neovascular age-related macular degeneration: ranibizumab vs bevacizumab. Eur J Ophthalmol. 2014;24:904–10.
Ghassemifar R, Lai CM, Rakoczy PE. VEGF differentially regulates transcription and translation of ZO-1alpha + and ZO-1alpha- and mediates trans-epithelial resistance in cultured endothelial and epithelial cells. Cell Tissue Res. 2006;323:117–25.
Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retinal Eye Res. 2013;34:19–48.
Terasaki H, Sakamoto T, Shirasawa M, et al. Penetration of bevacizumab and ranibizumab through retinal pigment epithelial layer in vitro. Retina. 2015;35:1007–15.
Kaur C, Foulds WS, Ling EA. Blood–retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retinal Eye Res. 2008;27:622–47.
Fontaine O, Olivier S, Descovich D, Cordahi G, Vaucher E, Lesk MR. The effect of intravitreal injection of bevacizumab on retinal circulation in patients with neovascular macular degeneration. Investig Ophthalmol Vis Sci. 2011;52:7400–5.
Barak A, Burgansky-Eliash Z, Barash H, Nelson DA, Grinvald A, Loewenstein A. The effect of intravitreal bevacizumab (Avastin) injection on retinal blood flow velocity in patients with choroidal neovascularization. Eur J Ophthalmol. 2012;22:423–30.
Micieli JA, Tsui E, Lam WC, Brent MH, Devenyi RG, Hudson C. Retinal blood flow in response to an intravitreal injection of ranibizumab for neovascular age-related macular degeneration. Acta Ophthalmol. 2012;90:e13–20.
Mottet B, Aptel F, Geiser MH, et al. Choroidal blood flow after the first intravitreal ranibizumab injection in neovascular age-related macular degeneration patients. Acta Ophthalmol. 2018;96:e783–8.
Segal O, Barayev E, Nemet AY, Mimouni M. Predicting response of exudative age-related macular degeneration to Bevacizumab based on spectralis optical coherence tomography. Retina. 2016;36:259–63.
Mathew R, Richardson M, Sivaprasad S. Predictive value of spectral-domain optical coherence tomography features in assessment of visual prognosis in eyes with neovascular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol. 2013;155:720–6.
Gamulescu MA, Panagakis G, Theek C, Helbig H. Predictive factors in OCT analysis for visual outcome in exudative AMD. J Ophthalmol. 2012:851648.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Gilda Cennamo, Daniela Montorio, Anna D’Alessandro, Pasquale Napolitano, Luca D’Andrea, Fausto Tranfa have no conflict of interest.
Compliance with Ethics Guidelines
The research protocol was approved by the Institutional Review Board of the University of Naples “Federico II” (PT 1124/14). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9825536.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cennamo, G., Montorio, D., D’Alessandro, A. et al. Prospective Study of Vessel Density by Optical Coherence Tomography Angiography After Intravitreal Bevacizumab in Exudative Age-Related Macular Degeneration. Ophthalmol Ther 9, 77–85 (2020). https://doi.org/10.1007/s40123-019-00221-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-019-00221-0