Abstract
Introduction
Combination use of atogepant and onabotulinumtoxinA has the potential to be more effective than either alone for the preventive treatment of chronic migraine (CM) due to their complementary mechanisms of action. This analysis collected real-world data to evaluate the safety, tolerability, and effectiveness of adding atogepant to onabotulinumtoxinA as a combination preventive treatment for CM.
Methods
This retrospective, longitudinal, multicenter chart review included adults with CM who received ≥ 2 consecutive cycles of onabotulinumtoxinA before ≥ 1 month of onabotulinumtoxinA and atogepant combination treatment. Charts at atogepant prescription (index date) and two subsequent onabotulinumtoxinA treatment visits (~ 3 and ~ 6 months post-index) were reviewed for change from baseline in monthly headache days (MHDs), ≥ 50% reduction in MHDs, discontinuation rates, and adverse events (AEs).
Results
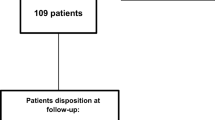
Of the 55 charts that met safety analysis criteria, 31 had data on headache days at index and first post-index visit and were eligible for effectiveness analysis (mean age 46.7 years, 94.5% female). For those with data available prior to onabotulinumtoxinA treatment (n = 25), the mean MHD was 24.0 days, reduced by 8.15 days after onabotulinumtoxinA treatment. After atogepant was added, MHD was incrementally reduced by 4.53 days and 8.75 days from index date to the first (N = 31) and second (N = 23) post-index onabotulinumtoxinA treatment visit, respectively. A ≥ 50% reduction in MHDs was achieved by 45.2% of patients ~ 3 months post-index. Atogepant and onabotulinumtoxinA were discontinued by 16.1% and 6.5% of patients, respectively. In the safety population, 32.7% of patients experienced ≥ 1 AE. No serious AEs were reported.
Conclusions
This real-world study of patients with CM demonstrated that adding atogepant to onabotulinumtoxinA as a combination preventive treatment for CM was effective by providing an additional reduction in MHDs over ~ 3 and ~ 6 months of combination treatment. Safety results were consistent with the known safety profiles of onabotulinumtoxinA and atogepant, with no new safety signals identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic migraine (CM) is a complex disease which may require combination therapy in individuals with continued burden after one preventive treatment. |
An American Headache Society position statement has noted that calcitonin gene-related peptide (CGRP) monoclonal antibodies could be combined with onabotulinumtoxinA for the preventive treatment of migraine, though the field lacks data on the combination of onabotulinumtoxinA and newer preventive CGRP treatments, such as gepants. |
Safety, tolerability, and effectiveness should be examined for the addition of atogepant to onabotulinumtoxinA for combination preventive treatment in migraine. |
What was learned from the study? |
The addition of atogepant treatment to patients receiving onabotulinumtoxinA for the preventive treatment of migraine provided an additional reduction in monthly headache days over 6 months of combination treatment. |
Safety results in patients who were treated with combination atogepant added to onabotulinumtoxinA for the prevention of CM were consistent with the known independent safety profiles of both onabotulinumtoxinA and atogepant. No new safety signals were identified. |
Introduction
Chronic migraine (CM) is a highly debilitating neurological disease characterized by the International Classification of Headache Disorders, 3rd edition (ICHD-3) as ≥ 15 headache days per month for 3 months over a 12-month period, with ≥ 8 headaches per month meeting migraine criteria [1]. Those with CM have frequent migraine attacks that impact daily life, with individuals reporting negative effects on relationships, work productivity, finances, and overall health [2, 3]. Preventive treatments can be used to manage CM and the American Headache Society (AHS) consensus statement states that preventive treatments should be considered for frequent attacks or attacks that significantly interfere with patients’ daily routines despite acute treatment [4]. Furthermore, a recent clinical perception paper acknowledged the importance of multimodal migraine management for CM, highlighting the importance of individualized treatment goals and effective multimodal management of migraine, which requires an understanding of the pathophysiology of migraine and how preventive treatments target these different physiological pathways [5].
OnabotulinumtoxinA has been approved for the preventive treatment of CM since 2010 and has established efficacy and safety from multiple clinical trials and real-world studies in individuals with CM [6,7,8,9,10]. OnabotulinumtoxinA is administered via injections into head and neck muscles which contain sensory neurons located in the trigeminal and cervical ganglia [11]. These areas of the peripheral nervous system have been well documented to play a role in the nociceptive, or sensory pain associated with a migraine attack. OnabotulinumtoxinA inhibits peripheral pain signaling to the brain by blocking the release of neurotransmitters and neuropeptides associated with pain and suppresses the peripheral sensitization by reducing cell surface expression of ion channels and sensory receptors [11,12,13]. Due to the mechanism of action of onabotulinumtoxinA and the complexity of CM pathophysiology, patients with CM who continue to experience frequent migraine attacks while receiving onabotulinumtoxinA may benefit from additional preventive treatment. An AHS position statement has noted that the calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) could be combined with onabotulinumtoxinA under specific criteria [14]. CGRP is a neuropeptide well documented as a potent vasodilator and inflammatory mediator that triggers migraine attacks [15, 16]. There have been several real-world studies evaluating CGRP mAbs and onabotulinumtoxinA combination for the preventive treatment of CM [17,18,19,20,21,22,23,24]. However, there is a lack of data for the evaluation of the addition of newer, oral migraine preventive treatment options, such as gepants, to onabotulinumtoxinA for the treatment of CM.
Gepants are a class of oral CGRP receptor antagonists and, to date, one prospective, real-world, observational study has evaluated the effectiveness of a gepant, ubrogepant, for the acute treatment of migraine in combination with onabotulinumtoxinA [25]. Atogepant is another member of the gepant class and is approved for the preventive treatment of migraine [26]. In preclinical models aimed at understanding the mode of action of onabotulinumtoxinA and atogepant, onabotulinumtoxinA preferentially inhibits unmyelinated C-meningeal nociceptor responses and attenuates the cortical spreading depression (CSD)-induced activation of wide-dynamic range (WDR) neurons, but not high-threshold (HT) neurons, in the spinal trigeminal nucleus (STN). Atogepant preferentially blocks thinly myelinated Aδ-meningeal nociceptors, which prevents the activation of HT neurons, but not WDR neurons, in the STN. Despite the inability of either agent to block both WDR and HT neurons, pre-clinical studies of combination onabotulinumtoxinA and atogepant demonstrated the prevention of sensitization of both WDR and HT neurons. These findings could be attributed to the partial inhibitory effect of onabotulinumtoxinA and atogepant on C- and Aδ-meningeal nociceptors, respectively, which block the CSD-induced activation and sensitization of nearly all trigeminovascular WDR and HT neurons in the STN [13, 27, 28]. Thus, dual blockade with onabotulinumtoxinA and atogepant may be more effective than monotherapy alone in reducing monthly headache days in individuals with CM due to the complementary mechanisms of action of the preventive treatments [28]. There have been no randomized trials to date evaluating the efficacy and safety of combination treatment with onabotulinumtoxinA and atogepant for the preventive treatment of CM.
We present here the first real-world evidence of atogepant added to onabotulinumtoxinA for the preventive treatment of CM. This retrospective chart review evaluated the safety, tolerability, and potential treatment benefits from real-world electronic medical records (EMR) of patients treated with continuous combination treatment with onabotulinumtoxinA and atogepant for ~ 6 months. Treatment benefits were based on effectiveness assessments that are widely used and recognized as being reliable and relevant to migraine.
Methods
Study Design
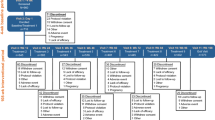
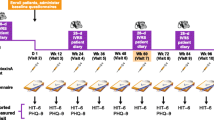
This multicenter, retrospective, longitudinal chart review study used data extracted from EMRs of eligible patients treated at five clinical sites in the United States: Los Angeles Headache Center—Los Angeles (Los Angeles, CA, USA), Los Angeles Headache Center—San Diego (San Diego, CA, USA), DENT Neurologic Institute (Buffalo, NY, USA), Diamond Headache Clinic (Chicago, IL, USA), and Neuroscience Group (Neenah, WI, USA), and had initiated the combination between November 1, 2021 and November 30, 2022. The index date was defined as the date of the onabotulinumtoxinA treatment visit at which atogepant was prescribed. A baseline period of 1–3 months prior to index was used to assess the effectiveness of onabotulinumtoxinA monotherapy treatment. Charts were reviewed up to 8 months prior to the index date to confirm inclusion criteria. Patients were followed for up to ~ 6 months post-index (corresponding to up to two onabotulinumtoxinA treatments; Fig. 1).
Study Population
Eligible patients were identified based on a physician’s diagnosis of CM in the patient chart and met the following criteria: ≥ 18 years of age, received ≥ 2 consecutive treatments with onabotulinumtoxinA prior to starting atogepant treatment, and received combination treatment for ≥ 1 month with onabotulinumtoxinA and atogepant (one cycle), initiated between November 1, 2021, and November 30, 2022. Patients were excluded if the time interval between onabotulinumtoxinA treatment and atogepant prescription was more than 4 weeks or if they received a CGRP treatment (monoclonal antibody or gepant) indicated for preventive use in migraine throughout the period between the last onabotulinumtoxinA treatment prior to the index date and the index date (~ 3 months), and if there were no migraine-specific treatments post index date. No a priori power or sample size calculations were performed as this was a descriptive study.
Data Collection
The site Principal Investigator (or their designee) identified potentially eligible patient charts at each site. Data were entered by staff under their supervision into an electronic case report form (eCRF) according to the protocol and study materials. The eCRF was made available in the electronic data capture (EDC) system Dacima (Montréal, QC, Canada). Dacima Clinical Suite is a secure, validated EDC software, and is fully compliant with FDA 21 CFR Part 11 requirements. The eCRF was developed to help optimize the collection of clean and homogeneous data by allowing for built-in data checks. An ‘Unknown’ option was made available in the EDC for variables where data may have been missing from the patient chart. This allowed for more complete data to be collected in the EDC by avoiding the option for variables to be left blank. Free-text entries were limited as much as possible in the eCRF to enhance data quality. Each site was provided with access to the secure online database in order to enter the data.
Baseline demographics, migraine-relevant clinical history, concomitant migraine medication(s) use, and duration of onabotulinumtoxinA treatment prior to starting atogepant treatment were collected. Treatment patterns were measured by onabotulinumtoxinA treatment dose and duration between treatment doses, the dose of atogepant prescribed, and any change of type, dose, or regimen.
Outcomes
Outcome measures to evaluate the potential benefits of combination treatment with onabotulinumtoxinA and atogepant were assessed at ~ 3 and 6 months post-index. Outcomes assessing treatment effectiveness included the mean change from baseline in monthly headache days (MHDs) at ~ 3 months and ~ 6 months and the proportion of participants with any, ≥ 25%, ≥ 50%, ≥ 75%, or 100% reduction from baseline in MHDs at ~ 3 and 6 months post-index.
Safety was evaluated by the frequency, types, and severity of adverse events (AEs) assessed from the patient charts. Treatment discontinuation was also evaluated by patient chart and categorized as discontinuation of onabotulinumtoxinA treatment, discontinuation of atogepant treatment, or concurrent discontinuation of both treatments and the reported reason for discontinuation when available.
Statistical Analyses
Statistical analyses were descriptive in nature and were conducted using SAS v9.04 and R version 4.1.3. For all analyses, continuous variables were summarized by mean, standard deviation (SD), median, range (i.e., minimum–maximum), and number and percentage of patients with unknown values. Categorical variables were summarized by number and percentage of patients in each possible category and those with unknown values. Additionally, the total number of patients at each visit and/or the total number of patients during follow-up were presented. Effectiveness outcomes and adverse events were described at the first (~ 3-month) and second (~ 6-month) post-index onabotulinumtoxinA treatment visits. Discontinuations were described cumulatively for the follow-up period for which the patient was receiving combination treatment.
Headache frequency recorded on a 90-day basis was converted to a 30-day basis at each follow-up interval. Distributions of the headache frequency converted from a 90-day to a 30-day basis were compared to those that were captured on a 30-day basis to ensure there were no statistically significant differences in distribution. If data were collected for a post-index visit dating more than 7.5 months post-index, this was considered an additional visit and not included in the analysis. Descriptive statistics related to headache frequency and mean change from baseline in headache day frequency are presented as days per month and were calculated for each follow-up visit.
This real-world retrospective study of the addition of atogepant to onabotulinumtoxinA treatment for CM was conducted on secondary data collected for the purpose of health care, not research. Missing values may be the result of several circumstances including data not being applicable for a specific patient, or data not being available in the medical chart. Not all data fields of study interest were available for each patient at a particular time point. Additionally, not all study patients contributed data to both post-index onabotulinumtoxinA administrations of study interest due to loss to follow-up or discontinuation. Missing data were captured explicitly by allowing for an unknown option in the eCRF wherever appropriate. The number and percentage of unknown values for a variable were reported. No imputation was performed, and all analyses were conducted on available data only, with the exception of date variables where day and/or month were missing, for which the first day of the month and/or the first month of the year were imputed, respectively. In case the sample size for exploratory variables was not sufficient, then the analyses were not reported.
Ethical Approval
The New England Independent Review Board reviewed the study protocol prior to study initiation and determined the study as exempt from review. Written informed consent from participants was not required for this study in accordance with the national legislation and the institutional requirements as only deidentified data were collected. The study was conducted in accordance with the International Conference for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki.
Results
Study Population
Medical records were provided by the five study sites for 77 patients deemed eligible (Supplementary Materials). Out of these patients, 22 patients were considered ineligible and were excluded from the analysis. The most common reason for exclusion was being on a preventive CGRP agent before index (N = 7) and having a time interval between onabotulinumtoxinA and atogepant treatment greater than 4 weeks (N = 6; Fig. 2). The safety population consisted of the remaining 55 patients, of which 31 patients had data on headache days at both the index and the first post-index onabotulinumtoxinA treatment visit and were included in the effectiveness population (Fig. 2). The safety population had a mean (SD) age of 46.58 (10.98) years and was predominantly female (94.5%). The mean (SD) time to index was 12.01 (10.94) years from migraine diagnosis, 5.34 (4.33) years from CM diagnosis, and 3.84 (2.49) years from onabotulinumtoxinA treatment initiation. During the 3-month baseline period prior to initiating atogepant, the majority of patients had comorbid conditions including 29.1% with anxiety, 23.6% with depression, and 65.5% of patients reporting unspecified comorbidities (Table 1). The most common acute medications utilized by the safety population were ubrogepant (50.9%), sumatriptan (29.1%), and rimegepant (23.6%; Supplementary Materials). The safety and effectiveness populations had similar baseline demographics (Table 1).
Combination Treatment Characteristics
Patients in the effectiveness population had a median dose of onabotulinumtoxinA of 155 U (range 155–200 U) across treatment sessions (Table 2). Most patients (96.8%) added atogepant 60 mg once daily (QD) to onabotulinumtoxinA treatment. The mean (SD) follow-up from index date to first post-index visit was 87.3 (9.4) days and 87.0 (9.4) days from the first to second post-index visit indicating that patients generally received onabotulinumtoxinA treatments at 3-month intervals, although with some considerable variation across the study population.
Treatment Effectiveness
Before onabotulinumtoxinA treatment, patients in the effectiveness population reported a mean MHD of 24.0 days (N = 25), with a mean change of − 8.15 days (95% CI: [– 11.44, – 4.85]) with onabotulinumtoxinA treatment (N = 25; Table 3). Most patients (68%) reported a reduction in MHDs after onabotulinumtoxinA treatment, with 60% reporting ≥ 25% reduction, 48% with ≥ 50% reduction, and 8% with ≥ 75% reduction. OnabotulinumtoxinA treatment resulted in 56.0% of the effectiveness population reporting < 15 MHDs, compared to 0% prior to onabotulinumtoxinA (Fig. 3). Data above is reflective of the subset of those patients with available headache frequency data prior to onabotulinumtoxinA treatment (N = 25) and baseline index data and not for the entire effectiveness population (N = 31).
Headache frequency data collected at index (start of atogepant) and at two onabotulinumtoxinA treatment visits post-index are presented in Table 4. The mean MHD at index was 17.88 days. At the first post-index onabotulinumtoxinA visit (~ 3 months, N = 31) there was a mean change of − 4.53 days (95% CI: [− 7.40, − 1.61]) in MHDs. At the second onabotulinumtoxinA visit (~ 6 months; N = 23) the mean change from index was − 8.75 days (95% CI: [− 13.21, − 4.29]) in MHDs (Fig. 4).
The percentage of patients who experienced any, ≥ 25%, ≥ 50%, ≥ 75%, or 100% reduction in MHDs increased from index to the first and second onabotulinumtoxinA treatment visits (Fig. 3; Table 4). The percentage of patients with any reduction in MHDs from index to the second onabotulinumtoxinA treatment visits was 81.0% (95% CI: [58.1, 94.6]), with more than half of patients (57.1%) exhibiting ≥ 50% reduction in MHDs, 33.3% exhibiting a ≥ 75% reduction in MHDs, and 9.5% of patients exhibiting a 100% reduction in MHDs (Fig. 3; Table 4).
The proportion of patients who achieved controlled CM (< 15 MHD) status on combination treatment increased from 48.4% at index to 61.3% at first visit post-index and 76.2% at second visit post-index (Supplementary Materials).
Safety and Tolerability
AEs reported over the entire follow-up duration or until patient discontinuation in the safety population are presented in Table 5. Throughout a mean (SD) follow-up period of 141.9 (51.4) days, 32.7% (18/55) of patients experienced ≥ 1 AE. Of these 18 patients, 11 experienced one AE, six experienced two AEs, and one experienced three AEs. The most common AEs (> 3%) were constipation (5.5%, 3/55), nausea (3.6%, 2/55), neck pain (3.6%, 2/55), fatigue (3.6%, 2/55), and abdominal discomfort (3.6%, 2/55). No serious AEs were reported.
Treatment Discontinuation
Discontinuation of atogepant and/or onabotulinumtoxinA treatment and changes in atogepant dosage during follow-up in the effectiveness and safety populations are presented in the Supplementary Materials. Overall, in the effectiveness population, two patients (6.5%, 2/31) discontinued onabotulinumtoxinA 84 days post-index, one due to lack of effect and one for other reasons. Five patients (16.1%, 5/31) discontinued atogepant from 87 to 126 days post-index, three due to lack of effect, one due to tolerability/safety, and one for unknown reasons. Two patients (6.5%) changed atogepant dose. Discontinuation results in the safety population (N = 55) were similar to the effectiveness population, with zero patients and one patient (6.7%) discontinuing onabotulinumtoxinA and atogepant due to tolerability/safety, respectively (Supplementary Materials).
Discussion
The safety and efficacy of onabotulinumtoxinA and atogepant have been established for the preventive treatment of CM [6,7,8,9,10]. However, due to the complex nature of CM, which involves multiple neuroanatomic pathways, vasoactive neuropeptides, and receptors, a personalized treatment plan may require the use of combination migraine preventive treatments for patients with continued significant migraine burden despite preventive treatments. The addition of a CGRP mAb to onabotulinumtoxinA treatment of CM is a commonly used practice by clinicians, although safety and efficacy data are limited [17, 19, 20]. This retrospective chart review of 55 patients across five sites within the US evaluated the safety and effectiveness of atogepant in combination with onabotulinumtoxinA for the preventive treatment of CM. The addition of atogepant to onabotulinumtoxinA was well tolerated, with no new safety signals identified, and was associated with additional clinical benefit. One-third of patients (32.7%) experienced ≥ 1 AE, with the most common AE being constipation (5.5%). Adverse events results are consistent with previous clinical trials and real-world studies of onabotulinumtoxinA or atogepant [29, 30]. Patients in the effectiveness population experienced a response to onabotulinumtoxinA prior to adding atogepant, consistent with the results of the phase 3 PREEMPT studies, and additional benefits after starting atogepant [30]. The addition of atogepant to onabotulinumtoxinA as a combination preventive treatment resulted in an incremental reduction in MHDs over ~ 6 months, with a mean change from baseline of − 4.5 days after ~ 3 months and – 8.75 days after ~ 6 months. Collectively, these results support that a combination of atogepant added to onabotulinumtoxinA for the preventive treatment of CM is likely to be well tolerated and provide clinical benefits.
Findings reported here were consistent with similar real-world studies which evaluated the effectiveness of CGRP mAbs when added to onabotulinumtoxinA. Multiple real-world, retrospective chart reviews have demonstrated that erenumab, fremanezumab, or galcanezumab added to onabotulinumtoxinA have added clinical benefit and no new safety signals for the preventive treatment of CM [17,18,19,20,21]. A retrospective chart review of patients receiving erenumab, fremanezumab, or galcanezumab added to onabotulinumtoxinA identified an incremental reduction in mean MHD post-index of − 3.78 days and − 4.60 days at 6 and 12 months post-index, respectively [18]. One observational study concluded there is improved benefit of erenumab combined with onabotulinumtoxinA compared to erenumab monotherapy for the preventive treatment of CM [22]. The results presented here add to this body of evidence of the additive clinical benefit of combining a preventive treatment with onabotulinumtoxinA. The combination of atogepant with onabotulinumtoxinA demonstrated additive clinical benefit and no new safety signals. Two patients discontinued onabotulinumtoxinA after index and five patients discontinued atogepant after index. All patients received at least two onabotulinumtoxinA treatments before the initiation of combination treatment. Patients not tolerating onabotulinumtoxinA were likely not included in study participation given they had previously discontinued, and this may account for the lower discontinuation rates of onabotulinumtoxinA. Collectively, these results support the design of a double-blind, randomized study adding preventive migraine treatments, including atogepant, to onabotulinumtoxinA.
These results should be interpreted with the following limitations. The medical records data used for these analyses was collected for the purpose of health care, not research. Therefore, some data may have not been available in the patient’s chart, or the data available was not captured by the data extraction process. Any missing data values, regardless of reason, were not imputed. Not all patients had 3 and 6 months of data due to loss of follow-up and discontinuation, which could introduce bias. Additionally, study outcomes are not consistently reported and the method for reporting varies. More real-world and controlled trials are needed to further assess safety and potential benefits of combination treatment. Treatment between patients may have varied as atogepant compliance is unknown, and onabotulinumtoxinA treatment is not always administered per label. Finally, the study size was smaller than expected, as many patients in clinical practice were excluded because they lacked a wash-out period between the end of treatment with CGRP mAbs and the initiation of atogepant, which prevented them from meeting study inclusion criteria.
Conclusions
These real-world data demonstrate that the combination use of atogepant added to onabotulinumtoxinA treatment for the preventive treatment of CM was well tolerated and no new safety signals were identified. There were additional benefits with the combination of atogepant and onabotulinumtoxinA treatment compared to onabotulinumtoxinA treatment alone. These analyses support a more controlled trial evaluating the combination of atogepant and onabotulinumtoxinA for the preventive treatment of CM.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The data and analyses generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. https://doi.org/10.1177/0333102417738202
Buse DC, Fanning KM, Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(8):1286–99. https://doi.org/10.1111/head.13613.
Lantéri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: A systematic review. Cephalalgia. 2010;31:837–50. https://doi.org/10.1177/0333102411398400.
Ailani J, Burch RC, Robbins MS, Board of Directors of the American Headache Society. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–39. https://doi.org/10.1111/head.14153.
Blumenfeld AM, Lipton RB, Silberstein S, et al. Multimodal migraine management and the pursuit of migraine freedom: a narrative review. Neurol Ther. 2023;12:1533–51. https://doi.org/10.1007/s40120-023-00529-x.
Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803. https://doi.org/10.1177/0333102410364676.
Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Adams AM. Long-term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13. https://doi.org/10.1186/s10194-018-0840-8.
Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–14. https://doi.org/10.1177/0333102410364677.
Ahmed F, Gaul C, García-Moncó JC, Sommer K, Martelletti P. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20:26. https://doi.org/10.1186/s10194-019-0976-1.
Rothrock JF, Adams AM, Lipton RB, Silberstein SD, Jo E, Zhao X, et al. FORWARD study: evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache. 2019;59:1700–13. https://doi.org/10.1111/head.13653.
Burstein R, Blumenfeld AM, Silberstein SD, Adams AM, Brin MF. Mechanism of action of onabotulinumtoxinA in chronic migraine: a narrative review. Headache. 2020;60:1259–72. https://doi.org/10.1111/head.13849.
Aurora SK, Brin MF. Chronic migraine: an update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache. 2017;57:109–25. https://doi.org/10.1111/head.12999.
Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014;34:853–69. https://doi.org/10.1177/0333102414527648.
Ailani J, Blumenfeld AM. Combination CGRP monoclonal antibody and onabotulinumtoxinA treatment for preventive treatment in chronic migraine. Headache. 2022;62:106–8. https://doi.org/10.1111/head.14244.
Arulmani U, Maassenvandenbrink A, Villalón CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–30. https://doi.org/10.1016/j.ejphar.2004.07.035.
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. https://doi.org/10.1152/physrev.00034.2015.
Blumenfeld AM, Frishberg BM, Schim JD, et al. Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther. 2021;10:809–26. https://doi.org/10.1007/s40122-021-00264-x.
Mechtler L, Saikali N, McVige J, Hughes O, Traut A, Adams AM. Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to onabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front Neurol. 2022;12: 788159. https://doi.org/10.3389/fneur.2021.788159.
Armanious M, Khalil N, Lu Y, Jimenez-Sanders R. Erenumab and onabotulinumtoxinA combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J Pain Palliat Care Pharmacother. 2021;35:1–6. https://doi.org/10.1080/15360288.2020.1829249.
Cohen F, Armand C, Lipton RB, Vollbracht S. Efficacy and tolerability of calcitonin gene–related peptide–targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med. 2021;22:1857–63. https://doi.org/10.1093/pm/pnab093.
Ozudogru SN, Bartell JW, Yuan H, Digre KB, Baggaley SK. The effect of adding calcitonin gene-related peptide monoclonal antibodies to onabotulinum toxin A therapy on headache burden: a retrospective observational case series. Headache. 2020;60:1442–3. https://doi.org/10.21203/rs.2.22760/v1.
Boudreau GP. Treatment of chronic migraine with erenumab alone or as an add on therapy: a real-world observational study. Anesth Pain Res. 2020;4:1–4. https://doi.org/10.33425/2639-846X.1037.
Yuan H, Cohen F, Driessen MT, et al. Real-world effectiveness of add-on fremanezumab in patients receiving onabotulinumtoxinA for the prevention of chronic migraine in a US tertiary headache center: a retrospective chart review study. Cephalalgia Rep. 2024. https://doi.org/10.1177/25158163241238448.
Scuteri D, Tonin P, Nicotera P, et al. Pooled analysis of real-world evidence supports anti-CGRP mabs and onabotulinumtoxinA combined trial in chronic migraine. Toxins. 2022;14:529. https://doi.org/10.3390/toxins14080529.
Adams AM, Hutchinson S, Engstrom E, et al. Real-world effectiveness, satisfaction, and optimization of ubrogepant for the acute treatment of migraine in combination with onabotulinumtoxinA: results from the COURAGE Study. J Headache Pain. 2023;24:102. https://doi.org/10.1186/s10194-023-01622-0.
QULIPTA [atogepant]. Package Insert. AbbVie Inc.: 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf. Accessed 15 Apr 2024.
Strassman AM, Melo-Carrillo A, Houle TT, Adams A, Brin MF, Burstein R. Atogepant – an orally-administered CGRP antagonist – attenuates activation of meningeal nociceptors by CSD. Cephalalgia. 2022;42:933–43. https://doi.org/10.1177/03331024221083544.
Melo-Carrillo A, Strassman AM, Schain AJ, Adams AM, Brin MF, Burstein R. Combined onabotulinumtoxinA/atogepant treatment blocks activation/sensitization of high-threshold and wide-dynamic range neurons. Cephalalgia. 2020;41:17–32. https://doi.org/10.1177/0333102420970507.
Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. New Engl J Med. 2021;385(8):695–706. https://doi.org/10.1056/nejmoa2035908.
Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–36. https://doi.org/10.1111/j.1526-4610.2010.01678.x.
Acknowledgements
AbbVie and authors thank all investigators and the patients who participated in this study.
Medical Writing and Editorial Assistance
Medical writing assistance was provided to the authors by Gabriel Jensen, PhD, and Brian Neel, PhD, of AbbVie (North Chicago, IL, USA), and editorial assistance was provided to the authors by Angela T. Hadsell, BA, of AbbVie, and was funded by AbbVie.
Funding
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. The journal’s Rapid Service Fee was funded by AbbVie. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the interpretation of the results, the writing, editing, review, and approval of the manuscript, including the visualization of the published data. Andrew M. Blumenfeld, Laszlo Mechtler, Lisa Cook, Christopher Rhyne, Brian Jenkins, and Merle Diamond contributed to the conceptualization of the study and/or the study execution. Brett Dabruzzo and Aubrey Manack Adams contributed to the conceptualization of the study, the methodology, supervision of the study, and project administration. Olivia Hughes contributed to the conceptualization of the study and performed the formal analysis.
Corresponding author
Ethics declarations
Conflict of Interest
Andrew M. Blumenfeld has served on advisory boards Allergan, Aeon, Alder, Amgen, Biohaven, Lilly, Lundbeck, Novartis, Pfizer, Revance, Teva, Theranica, Tonix. AB has been a speaker for Allergan/AbbVie, Lilly, Lundbeck, Teva, Theranica. AB has consulted for Aeon, Allergan/AbbVie, Alder, Amgen, Biohaven, Lilly, Lundbeck, Pfizer, Novartis, Teva, Theranica, Tonix. AB has received grand support from Aeon, Allergan/AbbVie, Amgen, Lundbeck, Revance. AB has been a contributing author for Aeon, Allergan/AbbVie, Amgen, Biohaven, Lilly, Lundbeck, Novartis, Teva, Theranica. AB has received stock options from Haven Health Inc. and Aeon. Andrew Blumenfeld is an Editorial Board member of Pain and Therapy. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Laszlo Mechtler has served on advisory boards for, consulted for, and/or been a speaker for, Amgen, Novartis, Allergan/AbbVie, Eli Lilly, Teva, Biohaven, Lundbeck, The institution of Dr. Mechtler has received research support from The Harry Dent Family Foundation, Inc., Amgen/ Novartis, Alder/ Lundbeck, AbbVie/ Allergan, Miles for Migraine, and American Migraine Foundation. Lisa Cook has served on advisory boards for, consulted for and/or been a speaker for AbbVie/Allergan, Impel, Biohaven and Teva. Christopher Rhyne has served on advisory boards for, consulted for, and/or been a speaker for AbbVie/Allergan, Amgen, Biohaven, Diamond Headache Clinic Research and Education Foundation, Lilly, Lundbeck, National Headache Foundation, Teva, and Theranica. Brian Jenkins has served on advisory boards for, consulted for, and/or been a speaker for Amgen, Novartis, Allergan/AbbVie, Eli Lilly, Teva, Biohaven, Lundbeck, Relivion. Brian Jenkins has received stock or an ownership interest from Prevacus and NeoCorTechs. Merle Diamond has served on advisory boards for, consulted for, served as an editor for, and/or been a speaker or contributing author for Allergan/AbbVie, Amgen, Assertio, Axsome Pharma, Eli Lilly, Impel NeuroPharma, Lundbeck, Promius Pharma, Supernus, Upsher-Smith, Teva. An immediate family member of Merle Diamond has received compensation for serving on a Scientific Advisory or Data Safety Monitoring board for Supernus and Teva. Olivia Hughes is an employee of ICON plc. Aubrey Manack Adams and Brett Dabruzzo are employees of AbbVie and may hold AbbVie stock.
Ethical Approval
The New England Independent Review Board reviewed the study protocol prior to study initiation and determined the study as exempt from review. Written informed consent from participants was not required for this study in accordance with the national legislation and the institutional requirements as only deidentified data were collected. The study was conducted in accordance with the International Conference for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki.
Additional information
Prior Presentation These analyses have been presented as a poster presentation at the 2024 annual meeting of the American Academy of Neurology (April 13–18, 2024, Denver, CO, USA).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Blumenfeld, A.M., Mechtler, L., Cook, L. et al. Real-World Evidence of the Safety and Effectiveness of Atogepant Added to OnabotulinumtoxinA for the Preventive Treatment of Chronic Migraine: A Retrospective Chart Review. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00649-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40122-024-00649-8