Abstract
Introduction
Closed-loop spinal cord stimulation (CL-SCS) is a recently introduced system that records evoked compound action potentials (ECAPs) from the spinal cord elicited by each stimulation pulse and uses this information to automatically adjust the stimulation strength in real time, known as ECAP-controlled SCS. This innovative system compensates for fluctuations in the distance between the epidural leads and the spinal cord by maintaining the neural response (ECAP) at a predetermined target level. This data collection study was designed to assess the performance of the first CL-SCS system in a real-world setting under normal conditions of use in multiple European centers. The study analyzes and presents clinical outcomes and electrophysiological and device data and compares these findings with those reported in earlier pre-market studies of the same system.
Methods
This prospective, multicenter, observational study was conducted in 13 European centers and aimed to gather electrophysiological and device data. The study focused on the real-world application of this system in treating chronic pain affecting the trunk and/or limbs, adhering to standard conditions of use. In addition to collecting and analyzing basic demographic information, the study presents data from the inaugural patient cohort permanently implanted at multiple European centers.
Results
A significant decrease in pain intensity was observed for overall back or leg pain scores (verbal numerical rating score [VNRS]) between baseline (mean ± standard error of the mean [SEM]; n = 135; 8.2 ± 0.1), 3 months (n = 93; 2.3 ± 0.2), 6 months (n = 82; 2.5 ± 0.3), and 12 months (n = 76; 2.5 ± 0.3). Comparison of overall pain relief (%) to the AVALON and EVOKE studies showed no significant differences at 3 and 12 months between the real-world data release (RWE; 71.3%; 69.6%) and the AVALON (71.2%; 73.6%) and EVOKE (78.1%; 76.7%) studies. Further investigation was undertaken to objectively characterize the physiological parameters of SCS therapy in this cohort using the metrics of percent time above ECAP threshold (%), dose ratio, and dose accuracy (µV), according to previously described methods. Results showed that a median of 90% (40.7–99.2) of stimuli were above the ECAP threshold, with a dose ratio of 1.3 (1.1–1.4) and dose accuracy of 4.4 µV (0.0–7.1), based on data from 236, 230, and 254 patients, respectively. Thus, across all three metrics, the majority of patients had objective therapy metrics corresponding to the highest levels of pain relief in previously reported studies (usage over threshold > 80%, dose ratio > 1.2, and error < 10 µV).
Conclusions
In conclusion, this study provides valuable insights into the real-world application of the ECAP-controlled CL-SCS system, highlighting its potential for maintaining effective pain relief and objective neurophysiological therapy metrics at levels seen in randomized control trials, and potential for quantifying patient burden associated with SCS system use via patient–device interaction metrics.
Clinical Trial Registration
In the Netherlands, the study is duly registered on the International Clinical Trials Registry Platform (Trial NL7889). In Germany, the study is duly registered as NCT05272137 and in the United Kingdom as ISCRTN27710516 and has been reviewed by the ethics committee in both countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A novel closed-loop spinal cord stimulation system (CL-SCS) is presented that records evoked compound action potentials from the spinal cord elicited by each stimulation pulse and uses this information to automatically adjust the stimulation strength in real time. |
This data collection study was designed to assess the performance of the first CL-SCS system in a real-world setting under normal conditions of use in multiple European centers. The study analyzes and presents clinical outcomes and electrophysiological and device data and compares these findings with those reported in earlier pre-market studies of the same system. |
The present results demonstrate that pain relief is sustained over a 12-month period and confirm the results of the prospective, multicenter single-arm AVALON study and the double-blind, multicenter, randomized controlled EVOKE study. |
Results presented in this study include three objective neural indicator metrics which describe the therapy dose, therapy utilization above the neural activation threshold, and the accuracy of CL-SCS therapy. |
Introduction
Spinal cord stimulation (SCS), a technique developed over five decades ago [1], has gained recognition as an effective pain management intervention for diverse medical conditions [2,3,4,5,6,7]. SCS leads are percutaneously implanted in the posterior epidural space, specifically over the dorsal aspect of the spinal cord, where myelinated axons of sensory nerves ascend in the dorsal columns. By applying electric currents to contacts on these leads, axons within the dorsal columns are activated, generating evoked compound action potentials (ECAPs) characterized by positive peaks (P1 and P2) and a negative peak (N1) [8, 9]. The size and shape of these peaks indicate the amount and type of axons being stimulated and their location relative to the lead [8, 9]. However, patient motion-related macro- or micro-movements, such as heartbeat, respiration, or cough-reflex, can affect the distance of the lead relative to the spinal cord, resulting in over- or under-stimulation in traditional open-loop SCS systems.
A closed-loop SCS (CL-SCS) system has recently been developed, which records ECAPs from the spinal cord elicited by each stimulation pulse and uses this information to automatically adjust the stimulation strength in real time, known as ECAP-controlled SCS [3, 10,11,12,13]. This innovative system compensates for fluctuations in the distance between the epidural leads and the spinal cord by maintaining the neural response (ECAP) at a predetermined target level. Specifically, the system achieves this by automatically adjusting stimulation output at a rate of 1800 times per minute at 30 Hz.
This CL-SCS system, the first of its kind, has received approval in Europe, Australia, and the United States for managing chronic neuropathic pain in the trunk and/or limbs. Its efficacy has been studied in two prospective trials—the EVOKE double-blind, multicenter, randomized controlled trial, and the AVALON prospective, single-arm, multicenter study [3, 10,11,12, 14].
Randomized controlled trials provide the highest level of evidence regarding the safety and efficacy of a treatment modality, but “real-world” evidence from registries and case-series are important for understanding how outcomes translate in uncontrolled clinical settings [15,16,17,18,19].
This data collection study was designed to assess the performance of the first CL-SCS system in a real-world setting under normal conditions of use in multiple European centers. The study analyzes and presents clinical outcomes and electrophysiological and device data and compares these findings with those reported in earlier pre-market studies of the same system.
Methods
Study Design
This prospective, multicenter, observational study was conducted in 13 European centers and aimed to gather electrophysiological and device data utilizing the Evoke® CL-SCS system (Saluda Medical, Bloomington, MN, USA). The study focused on the real-world application of this system in treating chronic pain affecting the trunk and/or limbs, adhering to standard conditions of use [20]. In addition to collecting and analyzing basic demographic information, the study presents data from the inaugural patient cohort permanently implanted at multiple European centers.
The observational data collection strictly adhered to the ethical principles outlined in the Declaration of Helsinki.
In the Netherlands, prospective data collection where people are only subjected to treatment in the context of their medical condition and not treatment in the context of the study falls outside the scope of the Medical Research Involving Human Subjects Act (WMO). Consequently, no ethics committee approval was required for the Dutch centers participating in this non-interventional study; however, an affirmation was provided by the ethics committee (Amsterdam University Medical Centers, W17_309#17.362). The study is duly registered on the International Clinical Trials Registry Platform (Trial NL7889). In Germany, the study is duly registered as NCT05272137 and in the United Kingdom as ISCRTN27710516 and has been reviewed by the ethics committees in both countries.
In accordance with the General Data Protection Regulation (GDPR), written informed consent was sought from all participating patients for the use of their data in the study.
Patients
The patients were informed about the data collection at the outpatient pain clinic of the 13 participating centers. The patients had to provide written informed consent before their data could be used. All candidates eligible for neuromodulation (male and female, ≥ 18 years, suffering from chronic pain in the trunk and/or limbs refractory to conservative therapy) were eligible for the data collection. There were no additional inclusion and exclusion criteria for the patients to be considered for this data collection beyond those routinely used to select patients as potential SCS candidates [20].
Settings
Patients underwent permanent SCS implantation between July 2019 and February 2023, with standard-of-care follow-up visits conducted at 3-month, 6-month, and 12-month intervals post-device activation. In instances where a standard follow-up visit could not be performed or patient outcomes were not obtained due to factors such as time constraints or the impact of the COVID-19 pandemic, these situations were not considered protocol deviations, and no assumptions were made regarding missing data.
The implantation of the CL-SCS system adhered to standard surgical procedures [20]. During the procedure, one or two percutaneous leads were implanted in the posterior epidural space over the dorsal columns, following conventional clinical practices for SCS. Intraoperative testing, known as sensation mapping, was conducted to confirm stimulation sensation in dermatomes associated with primary pain prior to lead fixation.
The neuromodulation system utilized in this study is an implantable device capable of both stimulating and recording electrophysiological data from the spinal cord. It offers two modes: ECAP-dose-controlled CL-SCS and fixed-output open-loop SCS. The system records ECAPs and can be used to inform programming in either stimulation mode.
The study center clinicians conducted programming, often with support from a field clinical engineer from the manufacturer (Saluda Medical), following standard clinical practices. Therapy optimization for each patient was achieved through a combination of electrophysiological recordings and patient feedback.
Data
Outcomes
The primary objective of this data collection study was to evaluate the performance of ECAP-controlled CL-SCS in a real-world setting. This assessment employed patient-reported outcomes (verbal numerical rating score [VNRS]: no pain = 0, worst possible pain = 10) and device data (ECAP recordings). Additionally, the study gathered data on subject satisfaction, stimulation awareness, and various neurophysiological measures. Stimulation awareness was categorized using response options ranging from always aware of stimulation sensation to never aware of stimulation sensation. Patient satisfaction was categorized using response options ranging from very satisfied to very unsatisfied.
Baseline characteristics, demographic data, and patient-reported outcomes were collected at the study’s commencement. This included information such as sex, height (cm), weight (kg), etiology, duration of chronic pain, nature of pain, pain intensity (VNRS), and previous neuromodulation.
During standard-of-care follow-up visits, data and patient-reported outcomes including pain intensity (VNRS), stimulation awareness, and patient satisfaction were collected.
Device Data
Electrophysiological and device data captured using the SCS system included ECAP size and morphology (amplitude, µV), device settings/parameters [e.g., current output (mA), pulse width (µs), frequency (Hz)], and patient interactions with their controller, outside of charging the controller (e.g., starting/stopping stimulation, changing programs, changing stimulation amplitude), and patient usage of the device (%).
Activation plots, collected at each follow-up visit, provided insights into the relationship between stimulation strength and spinal cord activation in each patient. These plots included ECAP amplitude and the patient’s subjective rating of perceived stimulation intensity.
To objectively characterize the neurophysiological SCS parameters in this study, objective therapy data were extracted from each subject’s clinic visit to their time of archival (August 2023), and neural metrics of dose ratio, percent time above ECAP threshold, and dose accuracy were calculated using previously described methods [21]. Associations were also explored between these metrics. Percent time above ECAP threshold, calculated as the percentage of time that stimulation was on and elicited dorsal column activation, was used as it has been shown to be more closely related to therapeutic efficacy than the percentage of total time that the device is active [21]. The dose ratio serves as a standardized measure of therapy dose, facilitating comparative analyses of dosage levels across patients [22]. It was calculated by matching the median ECAP amplitude to the fitted curve of the activation plot and dividing the corresponding current by the ECAP threshold to obtain a ratio. Ratios above 1 correspond to therapy levels above the ECAP threshold, and ratios below 1 indicate therapy was below the ECAP threshold. Dose accuracy is calculated as the root mean squared error between the target and measured activation levels. In open-loop therapy, such error tends to be considerable, as deviations in stimulation delivery from the target can arise from even minor physiological movements such as heartbeat and breathing. Conversely, CL-SCS therapy exhibits high stability, as the loop promptly adapts to changes in the distance between the electrodes and the spinal cord [21, 22].
Data Analysis
All data were automatically stored on the implanted device and downloaded to the clinical programmer for offline analysis.
Statistical analysis was conducted using GraphPad Prism software, calculating summary statistics including mean, median, standard error of the mean (SEM), and interquartile range (IQR, Q1 − Q3). VNRS data were presented in various formats, including raw scores, percentage change from baseline, responders, and high-responders. Patients who showed exceptional pain relief were defined as high-responders (≥ 80.0%) and patients who responded with ≥ 50.0% pain relief were defined as responders.
Non-parametric tests such as the Kruskal–Wallis test were applied to analyze VNRS data that did not follow normal distribution. Post hoc analysis utilized Dunn’s multiple comparisons test. For comparisons between real-world data, AVALON, and EVOKE, the Mann–Whitney U test was employed. The significance level was set at p < 0.05, and all values, excluding p-values, were rounded to one fractional digit.
Neural metrics data were analyzed using the non-parametric Spearman correlation coefficient.
Results
During the period from July 2019 to February 2023, a total of 148 new SCS patients at 13 centers were permanently implanted with the CL-SCS system based on the standard inclusion criteria for neuromodulation. The patients selected for the Evoke system represent the first use of this system worldwide following European marketing authorization.
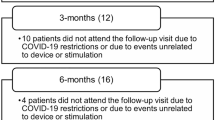
The COVID-19 pandemic led to significant restrictions in the usual follow-up examinations (standard of care); 45 patients were unable to participate in the 3-month examination, 57 patients were unable to participate in the 6-month examination, and 63 patients were unable to participate in the 12-month examination. If a follow-up examination was not performed or the patient results were not recorded due to time constraints, this was not considered a protocol deviation. No assumptions were made about missing data.
Baseline characteristics and demographics for all patients are presented in Table 1. A total of 121 patients had a diagnosis of chronic intractable neuropathic pain in the trunk and/or limbs due to persistent spinal pain syndrome after surgery (PSPS) type 2 [23]. Ten patients had a diagnosis of chronic intractable neuropathic pain of the trunk and/or limbs due to complex regional pain syndrome (CRPS). Seventeen patients had another diagnosis, including polyneuropathy, mononeuropathy, post-amputation pain, peripheral plexopathy, and nonsurgical back pain, among others.
Difference in sex: There were a greater number of female patients (n = 86) than male patients (n = 62) permanently implanted.
The number of patients in the different conditions included in the final analyses of the experimental outcomes is shown in Table 2. The three distinct conditions scrutinized encompass all patients, stimulation-naïve patients, and conversion patients. Stimulation-naïve patients include all individuals except those who had previously undergone implantation with an SCS device, whereas conversion patients are defined as individuals who had previously received treatment with at least one alternative SCS or dorsal root ganglion stimulator.
Pain Relief Outcomes: All Patients
Mean (± SEM) baseline (n = 148) overall pain scores (VNRS) were 8.2 ± 0.1. At 3 months (n = 103), average VNRS overall pain scores decreased to 2.6 ± 0.2, at 6 months (n = 91) to 2.7 ± 0.3, and at 12 months (n = 85) to 2.6 ± 0.2 (Fig. 1A). Analysis using the Kruskal–Wallis test revealed a significant change throughout all visits (Kruskal–Wallis test: H = 258.0, p < 0.0001, n = 427). Post hoc analyses (Dunn’s multiple comparisons test) demonstrated a significant improvement at all time points compared to baseline (baseline to 3 months: p < 0.0001; baseline to 6 months: p < 0.0001; baseline to 12 months: p < 0.0001). In addition, a twofold clinically meaningful change (minimum clinically important difference [MCID]; 2.0 [13]) at all follow-ups could be observed.
Patient-reported outcomes. A Mean (± SEM) baseline (n = 148) pain scores (VNRS) were 8.2 ± 0.1. After 12 months (n = 85), scores decreased to 2.6 ± 0.2. B Responder and high-responder rates. Vertical axis indicates responder/high-responder rates in %. Patients who showed exceptional pain relief were defined as high-responders (≥ 80.0%) and patients who responded with ≥ 50.0% pain relief were defined as responders. At 12 months, 76% of patients were responders and 41% high-responders. C Individual patient percentage change from baseline in overall pain scores at 12 months
Patients who showed exceptional pain relief were defined as high-responders (≥ 80.0%) and patients who responded with ≥ 50.0% pain relief were defined as responders. At 3 months the responder rate was 77% (n = 79 out of 103 patients), (≥ 50.0% pain relief), and the high-responder rate was 40% (n = 41 out of 103 patients), (≥ 80.0% pain relief). At 6 months, 81% (n = 74 out of 91 patients) of patients were responders, and 39% (n = 35 out of 91) of patients were high-responders. At 12 months, 77% (n = 65 out of 85) of patients were responders, and 41% (n = 35 out of 85) of patients were high-responders.
Sub-analysis of CRPS patients showed that mean (± SEM) baseline (n = 10) overall pain scores (VNRS) were 8.3 ± 0.4. At 3 months (n = 7), average VNRS overall pain scores decreased to 2.9 ± 1.3, at 6 months (n = 7) to 1.3 ± 0.5, and at 12 months (n = 7) to 2.3 ± 1.0.
Sub-analysis of PSPS patients showed that mean (± SEM) baseline (n = 121) overall pain scores (VNRS) were 8.2 ± 0.1. At 3 months (n = 88), average VNRS overall pain scores decreased to 2.4 ± 0.2, at 6 months (n = 75) to 2.8 ± 0.3, and at 12 months (n = 73) to 2.6 ± 0.3.
Pain Relief Outcomes: Stimulation-Naïve Patients
A sub-analysis was performed for stimulation-naïve patients (stimulation-naïve patients include all individuals except those who had previously undergone implantation with an SCS device; Table 2) for better comparison to the patient populations of the AVALON and EVOKE studies. Mean (± SEM) baseline (n = 135) overall pain scores (VNRS) were 8.2 ± 0.1. At 3 months (n = 93), average VNRS overall pain scores decreased to 2.3 ± 0.2, at 6 months (n = 82) to 2.5 ± 0.3, and at 12 months (n = 76) to 2.5 ± 0.3. Analysis using the Kruskal–Wallis test revealed a significant change throughout all visits (Kruskal–Wallis test: H = 244.4, p < 0.0001, n = 386). Post hoc analyses (Dunn’s multiple comparisons test) demonstrated a significant improvement at all time points compared to baseline (baseline to 3 months: p < 0.0001; baseline to 6 months: p < 0.0001; baseline to 12 months: p < 0.0001). In addition, a twofold clinically meaningful change (minimum clinically important difference [MCID]; 2.0 [13]) was observed at all follow-ups.
At 3 months, 81% (n = 75 out of 93 patients) were responders (≥ 50.0% pain relief) and 43% (n = 40 out of 93 patients) were high-responders (≥ 80.0% pain relief). At 6 months, 84% (n = 69 out of 82 patients) of patients were responders and 45% (n = 34 out of 82) of patients were high-responders. At 12 months, 80% (n = 61 out of 76) of patients were responders, and 45% (n = 34 out of 76) of patients were high-responders.
Percentage pain relief observed at 3 months and 12 months in this real-world cohort was 71% (n = 93) and 70% (n = 85), respectively. These outcomes were consistent with those reported for both the AVALON study [71% (n = 45); 74% (n = 43) pain relief at 3 and 12 months, respectively, post-implantation] and the EVOKE study [78% (n = 58); 77% (n = 55) pain relief at 3 and 12 months, respectively, post-implantation] (Fig. 2). The Mann–Whitney test results revealed no difference between the real-word data and the AVALON and the EVOKE for reported pain relief (%) at 3 months and 12 months.
Patient-reported outcomes—stimulation-naïve patients. A Mean (± SEM) baseline (n = 135) overall pain scores (VNRS) in stimulation-naïve patients were 8.2 ± 0.1. After 12 months, (n = 76) pain scores decreased to 2.5 ± 0.3. B Responder and high-responder rates. At 12 months, 80% (n = 61 out of 76) of patients were responders, and 45% (n = 34 out of 76) of patients were high-responders. C Individual patient percentage change from baseline in overall pain scores at 12 months. D Comparison of overall pain relief (%) to the AVALON and EVOKE studies. No significant differences at 3 and 12 months between the real-world data release (RWE; 71.3%; 69.6%) and the AVALON (71.2%; 73.6%) and EVOKE (78.1%; 76.7%) studies
Conversion Patients
Sub-analysis of conversion patients revealed that mean (± SEM) overall pain scores (VNRS) for salvage/conversion patients were 7.9 ± 0.3 at baseline (n = 13) (Fig. 3). At 3 months (n = 10), average VNRS overall pain scores decreased to 4.9 ± 0.9, at 6 months (n = 9) to 4.3 ± 1.1, and at 12 months (n = 9) to 3.8 ± 0.5. Analysis using the Kruskal–Wallis test revealed a significant change throughout all visits (Kruskal–Wallis test: H = 15.84, p < 0.0012, n = 41). Post hoc analyses (Dunn’s multiple comparisons test) demonstrated a significant improvement at all time points compared to baseline (baseline to 3 months: p < 0.0255; baseline to 6 months: p < 0.0085; baseline to 12 months: p < 0.0013). Mean (± SEM) baseline (n = 134) overall pain scores (VNRS) without salvage/conversion patients were 8.2 ± 0.1. At 3 months (n = 93), average VNRS scores decreased to 2.3 ± 0.2, at 6 months (n = 82) to 2.5 ± 0.3, and at 12 months (n = 76) to 2.5 ± 0.3.
Overview of prior neuromodulation experience and VNRS scores. A Sub-analysis of conversion patients (n = 13). Certain patients demonstrated prior use of multiple SCS devices. B Mean (± SEM) baseline (n = 13) overall pain scores (VNRS) for salvage/conversion patients were 7.9 ± 0.3. After 12 months (n = 9) pain scores decreased to 3.8 ± 0.5
Patient-Reported Outcomes: All Patients
Patient satisfaction data were collected during standard-of-care follow-up visits (Fig. 4A). At the 3-month visits, 94% (n = 90 out of 96) of the patients reported being very satisfied (58%, n = 56 out of 96) or satisfied (35%, n = 34 out of 96) with their therapy; 5% (n = 5 out of 96) of the patients reported being unsatisfied, and 1% (n = 1 out of 96) of the patients reported being very unsatisfied with the therapy. At 6 months, 93% (n = 83 out of 89) of the patients reported being very satisfied (57%, n = 51 out of 89) or satisfied (36%, n = 32 out of 89) with their therapy; 6% (n = 5 out of 89) of the patients reported being unsatisfied, and 1% (n = 1 out of 89) of the patients reported being very unsatisfied with the therapy. At 12 months, 92% (n = 78 out of 85) of the patients were very satisfied (60%, n = 51 out of 85) or satisfied (32%, n = 27 out of 85) with the ECAP-controlled CL-SCS therapy, whereas 5% (n = 4 out of 85) of the patients were unsatisfied and 4% (n = 3 out of 85) very unsatisfied.
Patient-reported outcomes—patient satisfaction and sensation awareness to stimulation. A Subjects rated their satisfaction with the stimulator using a four-point scale (with options ranging from “very unsatisfied” to “very satisfied”) at the 3-month, 6-month, and 12-month follow-ups. At the 3-month follow-up (n = 96), more than 95% of the patients were very satisfied or satisfied with the therapy, which only improved at the 12-month follow-up (n = 85), where the percentage of very satisfied patients increased even more. B Subjects rated their sensation awareness of the stimulator using a five-point scale (with options ranging from “never aware of stimulation sensation” to “always aware of stimulation sensation”) at the 3-month, 6-month, and 12-month follow-ups. More than 60% of all patients (n = 95) at the 3-month follow-up were either always aware or usually aware of the stimulation sensation. This amount stayed more or less the same at the 6-month and 12-month follow-up visits
Additionally, stimulation awareness was collected (Fig. 4B). At the 3-month visits, 42% (n = 40 out of 95) of the patients reported always being aware of the stimulation sensation; 23% (n = 22 out of 95) of the patients reported usually being aware of the stimulation sensation; 21% (n = 20 out of 95) of the patients reported sometimes being aware of the stimulation sensation; 12% (n = 11 out of 95) of the patients reported being rarely aware of the stimulation sensation; and 2% (n = 2 out of 95) of the patients reported never being aware of the stimulation sensation. At the 6-month visits, 25% (n = 22 out of 89) of the patients reported the always being aware of the stimulation sensation; 34% (n = 30 out of 89) of the patients reported usually being aware of the stimulation sensation; 30% (n = 27 out of 89) of the patients reported sometimes being aware of the stimulation sensation; 10% (n = 9 out of 89) of the patients reported being rarely aware of the stimulation sensation; and 1% (n = 1 out of 89) of the patients reported never being aware of the stimulation sensation. At the 12-month visits, 32% (n = 27 out of 84) of the patients reported always being aware of the stimulation sensation; 26% (n = 22 out of 84) of the patients reported usually being aware of the stimulation sensation; 26% (n = 22 out of 84) of the patients reported sometimes being aware of the stimulation sensation; 11% (n = 9 out of 84) of the patients reported being rarely aware of the stimulation sensation; and 5% (n = 4 out of 84) of the patients reported never being aware of the stimulation sensation.
Patient-Reported Outcomes: Stimulation-Naïve Patients
A sub-analysis was performed for stimulation-naïve patients. At the 3-month visits, 95% (n = 83 out of 87) of the patients reported being very satisfied (63%, n = 55 out of 87) or satisfied (32%, n = 28 out of 87) with their therapy; 3% (n = 3 out of 87) of the patients reported being unsatisfied and 1% (n = 1 out of 87) of the patients reported being very unsatisfied with the therapy. At 6 months, 95% (n = 76 out of 80) of the patients reported being very satisfied (60%, n = 48 out of 80) or satisfied (35%, n = 28 out of 80) with their therapy; 5% (n = 4 out of 80) of the patients reported being unsatisfied and 0% of the patients reported being very unsatisfied with the therapy. At 12 months, 92% (n = 70 out of 76) of the patients were very satisfied (63%, n = 48 out of 76) or satisfied (29%, n = 22 out of 76) with the ECAP-controlled CL-SCS therapy; 4% (n = 3 out of 76) of the patients reported being unsatisfied and 4% (n = 3 out of 76) of the patients reported being very unsatisfied with the therapy.
Additionally, stimulation awareness data were collected. At the 3-month visits, 43% (n = 37 out of 86) of the patients reported always being aware of the stimulation sensation; 21% (n = 18 out of 86) of the patients reported usually being aware of the stimulation sensation; 21% (n = 18 out of 86) of the patients reported sometimes being aware of the stimulation sensation; 13% (n = 11 out of 86) of the patients reported rarely being aware of the stimulation sensation; and 2% (n = 2 out of 86) of the patients reported never being aware of the stimulation sensation. At the 6-month visits, 26% (n = 21 out of 80) of the patients reported always being aware of the stimulation sensation; 35% (n = 28 out of 80) of the patients reported usually being aware of the stimulation sensation; 29% (n = 23 out of 80) of the patients reported sometimes being aware of the stimulation sensation; 9% (n = 7 out of 80) of the patients reported rarely being aware of the stimulation sensation; and 1% (n = 1 out of 80) of the patients reported never being aware of the stimulation sensation. At the 12-month visits, 31% (n = 23 out of 75) of the patients reported always being aware of the stimulation sensation; 27% (n = 23 out of 75) of the patients reported usually being aware of the stimulation sensation; 28% (n = 21 out of 75) of the patients reported sometimes being aware of the stimulation sensation; 9% (n = 7 out of 75) of the patients reported rarely being aware of the stimulation sensation; and 5% (n = 4 out of 75) of the patients reported never being aware of the stimulation sensation.
Recording and Characterizing ECAPs
ECAPs were successfully recorded across all patients, displaying the system’s capability to capture ECAPs at a specified frequency (e.g., 30 Hz = 30 times per second) and dynamically adjust to the real-time neurophysiological variations encountered by each patient throughout the day. In total, approximately 210 billion ECAPS were recorded from 85 patients during the 12-month period. As an example, 865,236,385 (0.86 billion) ECAPs were recorded from one particular patient during the course of 1 year.
Electrophysiological data, device parameters, and usage were analyzed to gain a better understanding of the prescribed therapy. Data for 1 week prior to the patient’s 3-month, 6-month, and 12-month visits were used for this analysis. Median values across the study cohort at 3 months, 6 months, and 12 months are shown for the in-clinic perception, comfort, and maximum. Out-of-clinic activation is summarized here by the mode ECAP amplitude [3, 12], with the estimated artefact removed [21]. If the activation plot was not analyzable due to technical issues or was not captured, the values were not taken into consideration. Therefore, not all patients have activation plot data presented here.
At 3 months, patients (n = 112) used their device 96% (median) of the time. The median stimulation frequency was 30 Hz and median pulse width was 320 µs (n = 89). Patients used their patient controller to adjust stimulation or program 1.1 (median) per day and charged their device 2.0 times (median) in the week prior to the visit (Fig. 5B). At 6 months, patients (n = 109) used their device 97% (median) of the time. The median stimulation frequency was 30 Hz and median pulse width was 300 µs (n = 79). Patients used their patient controller to adjust stimulation or program 0.7 (median) times per day. At 12 months, the patients (n = 98) used their device 97% (median) of the time and charged their device 2.0 times (median) per week. The median stimulation frequency was 30 Hz and median pulse width was 330 µs (n = 67). Patients used their patient controller to adjust stimulation or program once (median) every 3 days, i.e., 0.4 adjustments per day and charged their device 2.0 times (median) per week.
In-clinic and out-of-clinic spinal cord activation. A Median (IQR: Q1–Q3) data at 12 months (n = 52) revealed the ECAP threshold to be 2.7 µV (− 3.2 to 7.8). Median data at 12 months (n = 52) revealed the ECAP amplitude at perception threshold to be 6.0 µV (2.0–14.0). The ECAP amplitude at the maximum discomfort level was 55.0 µV (38.0–82.5) at 12 months. The mode ECAP amplitude target, the patients’ actual activation outside the clinic, was at every time point greater than the median ECAP threshold at each visit interval at 12.0 µV (0.0–28–6) at 12 months.*If the activation plot was not analyzable due to technical issues or was not captured, the values were not taken into consideration. Therefore, not all patients have activation plot data presented here. B Patients used their patient controller to adjust stimulation or program once (median) every 3 days, i.e., 0.4 adjustments per day
As part of standard procedure, activation plots were obtained for all patients during each follow-up visit (Fig. 5A). These plots were consistently gathered with the patient seated. They were generated incrementally to illustrate the correlation between current amplitude (mA) and the corresponding neural response (ECAP amplitude in µV). The individual sensitivity to stimulation (µV/mA) is described by the linear slope of the activation plot beyond the knee seen at threshold; at 3 months, patient sensitivity was 26.4 µV/mA, at 6 months 24.1 µV/mA, and at 12 months 30.5 µV/mA [8, 24].
Median (IQR: Q1–Q3) data at 3 months (n = 73), 6 months (n = 65), and 12 months (n = 52) revealed the ECAP threshold to be 2.7 µV (− 3.2 to 7.8) using 5.3 mA (3.8–6.3) at 3 months, 2.4 µV (− 6.6 to 7.6) using 5.4 mA (4.0–7.0) at 6 months, and 1.0 µV (− 3.7 to 7.2) using 5.6 mA (4.3–7.0) at 12 months. Median data at 3 months (n = 73), 6 months (n = 65), and 12 months (n = 52) revealed the ECAP amplitude at perception threshold to be 6.0 µV (2.0 – 14.0) using 5.0 mA (3.3–6.8) at 3 months, 6.0 µV (0–13.0) using 5.1 mA (3.1–7.0) at 6 months, and 8.5 µV (1.0–18.25) using 5.2 mA (3.7–6.6) at 12 months. The ECAP amplitude at the maximum discomfort level was 49.0 µV (29.5–72.5) using 7.2 mA (5.0–9.9) at 3 months (n = 71), 50.0 µV (35.0–73.0) using 7.5 mA (5.3–9.2) at 6 months (n = 65), and 55.0 µV (38.0–82.5) using 7.0 mA (5.1–9.8) at 12 months. The mode ECAP amplitude target, the patients’ actual activation outside the clinic, was at every time point greater than the median ECAP threshold at each visit interval: 14.6 µV (2.9–28.6) at 3 months (n = 74), 18.1 µV (6.1–28.2) at 6 months (n = 68), and 12.0 µV (0.0–28.6) at 12 months (n = 57). Patients were stimulating above their ECAP threshold 94% of the time at 3 months, 99% of the time at 6 months, and 99% of the time at 12 months.
Neural Metrics
Further investigation was undertaken to objectively characterize the physiological parameters of SCS therapy in this cohort using the metrics percent time above ECAP threshold (%), dose ratio, and dose accuracy (µV), using previously described methods [21, 22]. Results showed that a median of 90% (40.7–99.2) (data from n = 236 patients) of stimuli were above the ECAP threshold, with a dose ratio of 1.3 (1.1–1.4) (data from n = 230 patients) and dose accuracy of 4.4 µV (0.0–7.1) (data from n = 254 patients). Thus, across all three metrics, the majority of patients had objective therapy metrics corresponding to the highest levels of pain relief in previously reported studies (usage over threshold > 80%, dose ratio > 1.2, and error < 10 µV) [21, 22].
In order to gain deeper insights into the relationships among the three objective neural metrics, rank correlations were computed between each pair of metrics. The associations between metrics and the distributions of metric values are illustrated in Fig. 6. Spearman’s r2 value for the correlation between dose ratio and percentage of time above ECAP threshold is 0.9 (p < 0.0001), between dose ratio and dose accuracy is 0.5 (p < 0.001), and between percentage of time above ECAP threshold and dose accuracy is 0.4 (p < 0.0001).
Discussion
This prospective multicenter study, conducted in Europe, presents the real-world clinical outcomes, electrophysiological insights, and device data concerning the application of a novel ECAP-controlled CL-SCS system for the management of chronic pain.
Clinical outcomes from this study demonstrate sustained pain relief over a 12-month period, with a notable percentage of patients (stimulation-naïve: 45%) experiencing more than 80% pain relief, accompanied by high levels of patient satisfaction. These results align with outcomes observed in the AVALON prospective single-arm multicenter study and the EVOKE double-blind multicenter randomized controlled trial [3, 12]. Not all randomized controlled trials in SCS have translated into comparable outcomes in real-world cohorts [15, 16].
The ECAP-controlled CL-SCS system used in this study automatically adjusts stimulation output on every stimulus to maintain consistent therapeutic activation levels without causing over-stimulation-related side effects. This system contrasts with traditional open-loop SCS systems, which deliver fixed stimulation output without accounting for normal physical and physiological processes. The study introduces objective measures for patient experience, particularly the noteworthy metric of “button presses,” providing unique insight into patient burden associated with device interactions [25]. While SCS studies often lack objectivity in outcome measures, this study again addresses this limitation by presenting a median of 0.4 button presses per day, indicating minimal need for patient interaction.
Results presented in this study include three objective neural metrics which describe the therapy dose, therapy utilization above the neural activation threshold, and the accuracy of CL-SCS therapy. Relationships between these objective metrics and pain relief has previously been reported as initial evidence of a dose–response relationship in SCS [21, 22]. The fundamental metrics of therapy dose, usage above the ECAP threshold, and dose accuracy are inherently logical from first principles as essential measurable indicators for evaluating the utilization of an SCS stimulator and the performance of the stimulator in delivering therapeutic stimulation [21]. Usage metrics include the time the device is activated, as well as usage above the ECAP threshold as a percentage of time, highlighting the patient’s attempted and practical adherence to treatment. The neural dose ratio offers a normalized assessment of the effective therapeutic stimulation. The assessment of therapy variability can pinpoint exceptional cases where SCS operation is suboptimal, resulting in high variation in neural activation (administered dose) compared to the target dose; in such instances, reprogramming is advisable. The correlation between dosing levels and patient outcomes can serve as a guide for prescribing an appropriate dose level. Dose ratios below 1, indicating less than 50% usage over the ECAP threshold, typically result in minimal pain relief, likely attributed to inadequate stimulation of Aβ fibers [21]. Dose ratios equal to or exceeding 1.2 are expected to offer greater pain relief compared to peri-threshold or subthreshold stimulation levels. Furthermore, a dose ratio of 1.2 corresponds to the point at which the usage over threshold levels off to 100%, indicating a significant therapeutic threshold. Here, the correlation and metrics were comparable to previously reported data [21]. Even though the patient-reported outcomes were not taken into account the data indicates that across all three metrics, the majority of patients were categorized into groups corresponding to outcomes exceeding the median threshold (usage over threshold > 80%, dose ratio > 1.2, and error < 10 µV) [21, 22].
Limitations of the study include its lack of blinding and control arm, missing data due to pandemic-related visit cancellations, and some data gaps due to time constraints during clinic visits. The generalizability of findings is restricted by these limitations. Future studies should adopt a more systematic approach to device data collection, including comprehensive assessments of additional domains affected by chronic pain, such as quality of life and function. The study advocates for increased transparency in reporting SCS settings and patient–device interactions to facilitate meaningful comparisons across different systems and waveforms, aligning with recent recommendations by Katz and colleagues [18].
Conclusion
In conclusion, this study contributes valuable insights into the real-world application of the ECAP-controlled CL-SCS system, highlighting its potential for maintaining effective pain relief and objective neurophysiological therapy metrics at levels seen in randomized control trials, and potential for quantifying patient burden associated with SCS system use via patient–device interaction metrics.
With regard to future research, besides conducting randomized controlled trials for new indications and collecting big data in real-life studies/registries, studies should also focus on elucidating the mechanisms underlying the effectiveness of neuromodulation in general and CL-SCS in particular. An interesting question remains as to whether the ECAP can be used as a biomarker, potentially leading to an even more personalized application of neurostimulation. Finally, the utilization of clinical CL-SCS could enhance the implementation of preclinical SCS models by providing controlled and consistent activation of dorsal column fibers [26, 27].
Data Availability
The data was available for review by all the contributing authors. All data generated or analyzed during this study are included in this published article.
References
Shealy CN, Taslitz N, Mortimer JT, Becker DP. Electrical inhibition of pain: experimental evaluation. Anesth Analg. 1967;46(3):299–305.
Kumar K, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–88.
Mekhail N, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–34. https://doi.org/10.1016/S1474-4422(19)30414-4.
Duarte RV, Soliday N, Leitner A, Taylor RS. Health-related quality of life associated with pain health states in spinal cord stimulation for chronic neuropathic pain. Neuromodul Technol Neural Interface. 2020. https://doi.org/10.1111/ner.13267.
Duarte RV, et al. Spinal cord stimulation for the management of painful diabetic neuropathy: a systematic review and meta-analysis of individual patient and aggregate data. Pain. 2021;162(11):2635–43. https://doi.org/10.1097/j.pain.0000000000002262.
Eldabe S, et al. The effectiveness and cost-effectiveness of spinal cord stimulation for refractory angina (RASCAL study): a pilot randomized controlled trial: SCS for refractory angina (RASCAL study). Neuromodul Technol Neural Interface. 2016;19(1):60–70. https://doi.org/10.1111/ner.12349.
Petersen EA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687–98.
Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153(3):593–601. https://doi.org/10.1016/j.pain.2011.11.023.
Parker JL, et al. Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation. 2013;16(4):295–303. https://doi.org/10.1111/ner.12053. (discussion 303).
Brooker C, et al. ECAP-controlled closed-loop spinal cord stimulation efficacy and opioid reduction over 24-months: final results of the prospective, multicenter, open-label Avalon study. Pain Pract. 2021. https://doi.org/10.1111/papr.13008.
Mekhail N, et al. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurol. 2022;79(3):251–60. https://doi.org/10.1001/jamaneurol.2021.4998.
Russo M, et al. Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label Avalon study. Neurosurgery. 2020. https://doi.org/10.1093/neuros/nyaa003.
Levy RM, et al. Holistic treatment response: an international expert panel definition and criteria for a new paradigm in the assessment of clinical outcomes of spinal cord stimulation. Neuromodul Technol Neural Interface. 2023. https://doi.org/10.1016/j.neurom.2022.11.011.
Mekhail NA, et al. ECAP-controlled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Reg Anesth Pain Med. 2023. https://doi.org/10.1136/rapm-2023-104751.
De Andres J, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med Malden Mass. 2017;18(12):2401–21. https://doi.org/10.1093/pm/pnx241.
Deer T, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodul Technol Neural Interface. 2018;21(1):56–66. https://doi.org/10.1111/ner.12698.
Kapural L, et al. Novel 10-khz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–60. https://doi.org/10.1097/ALN.0000000000000774.
Katz N, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on methods, measurement, and pain assessment in clinical trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain. 2021;162(7):1935–56. https://doi.org/10.1097/j.pain.0000000000002204.
Schwartz JL. Real-world evidence, public participation, and the FDA. Hastings Cent Rep. 2017;47(6):7–8.
Deer TR, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for surgical technique for spinal cord stimulation. Neuromodul Technol Neural Interface. 2022;25(1):1–34.
Muller L, et al. First evidence of a biomarker-based dose-response relationship in chronic pain using physiological closed-loop spinal cord stimulation. Reg Anesth Pain Med. 2024. https://doi.org/10.1136/rapm-2024-105346.
Single PS, Scott JB, Mugan D. Measures of dosage for spinal-cord electrical stimulation: review and proposal. IEEE Trans Neural Syst Rehabil Eng. 2023;31:4653–60.
Christelis N, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med. 2021;22(4):807–18. https://doi.org/10.1093/pm/pnab015.
Parker J, Karantonis D, Single P. Hypothesis for the mechanism of action of ECAP-controlled closed-loop systems for spinal cord stimulation. Healthc Technol Lett. 2020;7(3):76–80. https://doi.org/10.1049/htl.2019.0110.
Nijhuis HJ, Hofsté W-J, Krabbenbos IP, Dietz BE, Mugan D, Huygen F. First report on real-world outcomes with evoked compound action potential (ECAP)-controlled closed-loop spinal cord stimulation for treatment of chronic pain. Pain Ther. 2023;12(5):1221–33.
Versantvoort EM, Dietz BE, Mugan D, Vuong QC, Luli S, Obara I. Evoked compound action potential (ECAP)-controlled closed-loop spinal cord stimulation in an experimental model of neuropathic pain in rats. Bioelectron Med. 2024;10(1):2.
Dietz BE, Mugan D, Vuong QC, Obara I. Electrically evoked compound action potentials in spinal cord stimulation: implications for preclinical research models. Neuromodul J Int Neuromodul Soc. 2022;25(1):64–74. https://doi.org/10.1111/ner.13480.
Acknowledgements
The study was sponsored by Saluda Medical. The authors gratefully acknowledge the Hospital Units for their support and assistance in this work and Weirong Ge for data analysis.
Funding
This study was sponsored and funded by Saluda Medical. The sponsor funded the publication review and the Rapid Service Fee. We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
Author information
Authors and Affiliations
Contributions
Harold Nijhuis conducted the study including data collection and analysis, conducted statistical analysis and interpretation of the data, and wrote the manuscript; Jan-Willem Kallewaard, Johan van de Minkelis, Willem-Jan Hofsté, Lars Elzinga, Philippa Armstrong, Ismaïl Gültuna, Emre Almac, Ganesan Baranidharan, Serge Nikolic, Ashish Gulve, Jan Vesper and Frank Huygen conducted the study including data collection and reviewed and edited the manuscript; Dave Mugan designed the study, interpreted the data and wrote the manuscript; Birte E. Dietz performed data and statistical analysis, interpretation of the data, and wrote the manuscript; All authors approved the final manuscript and agree with its submission.
Corresponding author
Ethics declarations
Conflict of Interest
Harold Nijhuis is a paid consultant for Saluda Medical, and Abbott. Harold Nijhuis has a research grant with Abbott. Willem-Jan Hofsté is a paid consultant for Saluda Medical and Abbott. Philippa Armstrong has received travel grants and speaker fees from Saluda Medical. Johan van de Minkelis is a paid consultant for Saluda Medical. Jan Vesper is a paid consultant for Abbott, Boston Scientific, UniQure, Curonix. Jan Vesper has research grants with Abbott and Boston Scientific and is on the advisory board of Abbott. Ashish Gulve is on the advisory board for Medtronic and Boston Scientific. Ashish Gulve has a research grant with Saluda Medical. Ashish Gulve reports personal fees unrelated to the submitted work from Nevro, Medtronic, Boston Scientific and Mainstay Medical. Jan-Willem Kallewaard is on the advisory board of Saluda Medical, Medtronic, Boston Scientific and Abbott. Jan-Willem Kallewaard has research grants from Boston Scientific, Abbott, Nevro and Saluda Medical. Frank Huygen reports personal fees from Abbott, grants, personal fees from Saluda, personal fees from Boston Scientific, personal fees from Grunenthal, personal fees from Pfizer, outside the submitted work. Serge Nikolic is a paid consultant for Saluda Medical, Nevro, and Stratus Medical. Birte E. Dietz and Dave Mugan are employed by Saluda Medical. There are no other relationships that might lead to a conflict of interest in the current study. All other authors have no conflicts of interest to declare.
Ethical Approval
The observational data collection strictly adhered to the ethical principles outlined in the Declaration of Helsinki. In the Netherlands, prospective data collection where people are only subjected to treatment in the context of their medical condition and not treatment in the context of the study falls outside the scope of the Medical Research Involving Human Subjects Act (WMO). Consequently, no ethics committee approval was required for the Dutch centers participating in this noninterventional study, an affirmation was provided by the ethics committee (Amsterdam University Medical Centers, W17_309#17.362). The study is duly registered on the International Clinical Trials Registry Platform (Trial NL7889). In Germany, the study is duly registered as NCT05272137 and in the United Kingdom as ISCRTN27710516 and has been reviewed by the ethics committee in both countries. In accordance with the General Data Protection Regulation (GDPR), written informed consent was sought from all participating patients for the use of their data in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nijhuis, H., Kallewaard, JW., van de Minkelis, J. et al. Durability of Evoked Compound Action Potential (ECAP)-Controlled, Closed-Loop Spinal Cord Stimulation (SCS) in a Real-World European Chronic Pain Population. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00628-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40122-024-00628-z