Abstract
Introduction
A novel closed-loop spinal cord stimulation (SCS) system has recently been approved for use which records evoked compound action potentials (ECAPs) from the spinal cord and utilizes these recordings to automatically adjust the stimulation strength in real time. It automatically compensates for fluctuations in distance between the epidural leads and the spinal cord by maintaining the neural response (ECAP) at a determined target level. This data collection was principally designed to evaluate the performance of this first closed-loop SCS system in a ‘real-world’ setting under normal conditions of use in a single European center.
Methods
In this prospective, single-center observational data collection, 22 patients were recruited at the outpatient pain clinic of the St. Antonius Hospital. All candidates were suffering from chronic pain in the trunk and/or limbs due to PSPS type 2 (persistent spinal pain syndrome). As standard of care, follow-up visits were completed at 3 months, 6 months, and 12 months post-device activation. Patient-reported outcome data (pain intensity, patient satisfaction) and electrophysiological and device data (ECAP amplitude, conduction velocity, current output, pulse width, frequency, usage), and patient interaction with their controller were collected at baseline and during standard of care follow-up visits.
Results
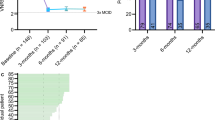
Significant decreases in pain intensity for overall back or leg pain scores (verbal numerical rating score = VNRS) were observed between baseline [mean ± SEM (standard error of the mean); n = 22; 8.4 ± 0.2)], 3 months (n = 12; 1.9 ± 0.5), 6 months (n = 16; 2.6 ± 0.5), and 12 months (n = 20; 2.0 ± 0.5), with 85.0% of the patients being satisfied at 12 months. Additionally, no significant differences in average pain relief at 3 months and 12 months between the real-world data (77.2%; 76.8%) and the AVALON (71.2%; 73.6%) and EVOKE (78.1%; 76.7%) studies were observed.
Conclusions
These initial ‘real-world’ data on ECAP-controlled, closed-loop SCS in a real-world clinical setting appear to be promising, as they provide novel insights of the beneficial effect of ECAP-controlled, closed-loop SCS in a real-world setting. The presented results demonstrate a noteworthy maintenance of pain relief over 12 months and corroborate the outcomes observed in the AVALON prospective, multicenter, single-arm study and the EVOKE double-blind, multicenter, randomized controlled trial.
Trial Registration
The data collection is registered on the International Clinical Trials Registry Platform (Trial NL7889).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A novel closed-loop spinal cord stimulation (SCS) system records evoked compound action potentials (ECAPs) from the spinal cord and uses these recordings to automatically adjust the stimulation intensity in real time. |
The objective of the data collection was to evaluate the performance of this first closed-loop SCS system in a ‘real-world’ setting under normal conditions of use in a single European center. |
A significant decrease in pain intensity was observed for overall back or leg pain scores were observed between baseline and follow-up visits, and patients were satisfied with the therapy. |
The initial data on ECAP-controlled closed-loop SCS in a real-world clinical setting are promising. |
The present results demonstrate that pain relief is sustained over a 12-month period and confirm the results of the prospective, multicenter, single-arm AVALON study and the double-blind, multicenter, randomized controlled EVOKE study. |
Introduction
Spinal cord stimulation (SCS) was developed over 50 years ago [1], and has been established as an effective treatment for relieving pain in patients with a variety of conditions [2,3,4,5,6,7]. SCS leads are implanted in the posterior epidural space over the dorsal aspect of the spinal cord where myelinated axons of sensory nerves ascend in the dorsal columns. The application of electric currents to contacts on the epidural leads subsequently activate axons within the dorsal columns to generate action potentials. The summation of all action potentials evoked by a given stimulus pulse is called an evoked compound action potential (ECAP). The ECAP has two positive peaks (P1 and P2) and one negative peak (N1) [8, 9]. The size and shape of these peaks are related to the amount and type of axons being stimulated, as well as their location with respect to the lead [8, 9]. The distance of the lead relative to the spinal cord is affected by patient motion-related macro- or micro-movements of physiologic origin, like heartbeat, respiration, or cough reflex. These movements can result in over- or under-stimulation in traditional open-loop SCS systems.
Recently, a novel closed-loop SCS system has been developed which records ECAPs from the spinal cord elicited by each stimulation pulse, and utilizes these recordings to automatically adjust the stimulation strength in real time (i.e., ECAP-controlled SCS) [3, 10]. This new system automatically compensates for fluctuations in the distance between the epidural leads and the spinal cord by maintaining the neural response (ECAP) at a determined target level. In more detail, the system maintains the determined target ECAP by automatically adjusting stimulation output (1800 times per minute at 30 Hz).
This is the first physiologic closed-loop-controlled SCS device approved in Europe that is indicated for the management of chronic pain in the trunk and/or limbs. This therapy has been studied in two prospective studies of level I and level II evidence, and has been shown to be effective in relieving pain. These studies include the EVOKE double-blind, multicenter, randomized controlled trial and the AVALON prospective, single-arm, multicenter study [3, 10,11,12].
Today, randomized controlled trials provide the highest level of evidence with respect to safety and efficacy of a treatment modality. However, ‘real-world’ evidence, generated from registries and case-series, provide valuable insights into the replicability of the randomized controlled trial outcomes in an uncontrolled clinical setting [13]. ‘Real-world’ data are also required to address questions about healthcare use and long-term safety of new therapeutic interventions [13, 14]. A closer review of randomized controlled trials published in the last decade makes it evident that reported outcomes are sometimes hard to replicate in a ‘real-world’ clinical environment [13, 15,16,17]. This data collection study was principally designed to evaluate the performance of this first closed-loop SCS system in a ‘real-world’ setting under normal conditions of use in a single European center. Clinical outcomes as well as electrophysiological and device data are analyzed and presented. We also compare these findings with those reported in earlier, pre-market studies of the same system.
Methods
Study Design
This prospective, observational, single-center data collection study was designed to collect electrophysiological and device data using the Evoke™ closed-loop SCS system (Saluda Medical, Sydney, Australia) for the treatment of chronic pain of the trunk and/or limbs in a ‘real-world’ setting under normal conditions of use. Additionally, basic demographic and outcome data were collected and analyzed. Here, the first patient cohort permanently implanted at St. Antonius Hospital, Nieuwegein, Netherlands, following European regulatory approval (CE Mark) is presented. This observational data collection adhered to the ethical principles of the Declaration of Helsinki. In the Netherlands, a prospective data collection without any intervention does not fall under the Medical Research Involving Human Subject Act. This means that no medical ethical committee approval is required for this type of study in the Netherlands, as confirmed by the ethical committee (Amsterdam University Medical Centers, W17_309#17.362). The data collection is registered on the International Clinical Trials Registry Platform (Trial NL7889). For the use of the data, we followed the general data protection regulations and asked for written informed consent from the patients.
Patients
The patients were informed about the data collection at the outpatient pain clinic of the St. Antonius Hospital. The patients had to provide written informed consent before their data could be used. All candidates eligible for neuromodulation (male and female, ≥ 18 years and suffering from chronic pain in the trunk and/or limbs due to PSPS type 2 (persistent spinal pain syndrome after surgery) refractory to conservative therapy) were eligible for the data collection. There were no additional inclusion and exclusion criteria for the patients to be considered for this data collection beyond those routinely used to select patients as potential SCS candidates.
Settings
Patients underwent permanent SCS implantation between August 2019 and September 2020. As standard of care, follow-up visits were completed at 3 months, 6 months, and 12 months post-device activation. If a standard of care follow-up visit was not performed or patient outcomes were not taken, for example due to time limitations or COVID-19, it was not regarded as a protocol deviation and no assumptions were made in relation to such missing data.
The implantation of the closed-loop SCS system was performed according to standard surgical procedures [18]. During the implant procedure, two percutaneous leads were implanted in the posterior aspect of the epidural space over the dorsal columns as per standard SCS clinical practice. Intra-operative testing was performed to confirm stimulation sensation in the dermatomes associated with primary pain prior to lead fixation in all patients, so-called sensation mapping.
The neuromodulation system used in this study (Evoke™ closed-loop SCS system; Saluda Medical) is an implantable, stimulating, and recording device that can measure and store electrophysiological data from the spinal cord. It offers both ECAP-controlled, closed-loop SCS and fixed-output, open-loop SCS. ECAPs can be recorded, as well as used to inform programming (i.e., ECAP-guided programming) in either stimulation mode.
The study center clinicians performed programming, usually with support from a field clinical engineer from the manufacturer (Saluda Medical) as per standard clinical practice. Therapy was optimized for each patient using both electrophysiological recordings and patient feedback.
Data
Outcomes
The primary purpose of this data collection study was to assess the performance of ECAP-controlled closed-loop SCS in a real-world setting. This was assessed using patient-reported outcomes (e.g., verbal numerical rating score; VNRS) and device data (ECAP recordings). Additionally, the data collection study captured data on subject satisfaction and various other neurophysiological measures (e.g., conduction velocity; CV). The following baseline characteristics, demographic data, and patient-reported outcomes were collected at baseline: age (years), sex, height (cm), weight (kg), etiology, duration of chronic pain, nature of pain (neuropathic or neuropathic-like, nociceptive, mixed), primary area of chronic pain and pain intensity (VNRS, worst possible pain = 10).
During standard of care follow-up visits, the following data and patient-reported outcomes were collected: reason for visit, pain intensity (VNRS), and patient satisfaction. Patient satisfaction data were collected using the following response options: 1. Very satisfied, 2. Satisfied, 3. Quite satisfied, 4. Quite unsatisfied, 5. Unsatisfied, or 6. Very unsatisfied.
Device Data
Electrophysiological and device data captured using the SCS system consisted of the size and morphology of the ECAP (amplitude, µV), CV (m/s), and device settings/parameters [e.g., current output (mA), pulse width (ms), frequency (Hz), and usage (%)], and patient interaction with their controller (captured interaction data include starting stimulation, stopping stimulation, changing programs, and changing amplitude of stimulation).
For all patients, activation plots were collected as standard of care at every follow-up visit. Activation plots were always collected with the patient in a seated position. Activation plots are generated by increasing the stimulation in a stepwise manner to describe the relationship between stimulation strength and spinal cord activation in each patient. During activation plot collection, the ECAP amplitude, charge delivered, and patient’s subjective rating of perceived stimulation intensity were collected as previously described [8, 19].
CV is the speed at which an ECAP signal propagates along the neural pathway (measured in m/s). CV was measured antidromically and orthodromically by measuring the ECAP propagation along the lead by stimulating on the most cephalad contact and measuring from multiple contacts beneath it (antidromic measurement), and stimulating at the most caudal contact, and then taking measurements from the contacts above (orthodromic measurement). CV measurements were performed using 10 Hz, 240 µs, and at a comfortable stimulation intensity, for comparison to previous recorded data [11].
Data Analysis
All data were automatically stored on the implanted device. During interrogation of the implanted device, data were downloaded to the clinical programmer and analyzed offline.
STATISTICA 13 software (Statsoft, Tulsa, USA) was used for statistical analysis. Summary statistics were calculated as appropriate, including means, medians, modes, standard errors of the mean (SEM), and interquartile ranges (IQR, Q1–Q3). VNRS data are presented as raw scores, percentage change from baseline, responders (≥ 50% pain relief), and high-responders (≥ 80% pain relief).
For statistical analysis, the data were first tested for normal distribution with Kolmogorov–Smirnov test. Data from VNRS did not follow normal distribution and were therefore analyzed using non-parametric tests. The non-parametric version of the one-way repeated measures ANOVA, Friedman’s ANOVA, was applied to test the significance through experiments. For post hoc analysis the Wilcoxon signed rank test was used. For comparison between the real-world data (AZN), AVALON, and EVOKE, the Mann–Whitney–U test was used. The level of significance was set at p < 0.05. All values excluding the p values were rounded to one fractional digit.
Results
During the period July 2019 and October 2020, a total of 35 new SCS patients underwent SCS implantation in Nieuwegein, 24 of whom were selected to trial the Saluda system based on standard inclusion criteria for neuromodulation in the Netherlands. Younger patients were preferentially selected as the team felt confident that these patients would cope well with a rechargeable system and would be unlikely to require MRI, as full body conditional MRI labeling was not immediately available. Of these 24 patients, 22 went on to receive the fully implantable system (two unsuccessful trials). Of these 22 patients, 20 were followed-up at 12-month post-implant, one died due to an unrelated adverse event (cancer), and one was diagnosed with a neuroblastoma and was lost to follow-up. COVID-19 caused significant restrictions to usual (standard of care) follow-up visits; ten patients were not able to attend the 3-month visit and six patients did not attend the 6-month visit. If a follow-up visit was not performed, or patient outcomes were not taken due to time limitations, it was not regarded as a protocol deviation. No assumptions were made for missing data. Apart from the deceased patient, none of the 22 patients were explanted (see Fig. 1).
Study design and flowchart. A total of 22 patients were permanently implanted, of whom 20 completed their 12-month visit. One patient died due to an unrelated adverse event (cancer), and one was diagnosed with a neuroblastoma and was lost to follow-up. If a follow-up visit was not performed, or patient outcomes were not taken due to time limitations, it was not regarded as a protocol deviation
Baseline characteristics and demographics for all patients are presented in Table 1. All patients had a diagnosis of chronic intractable pain of the trunk and/or limbs due to failed back surgery syndrome (FBSS), referred to now as PSPS type 2. Fifteen patients had predominantly pain in the leg, five predominantly pain in the buttock, and the remaining two having predominantly back pain. There were a greater number of females (n = 14) than males (n = 8) permanently implanted.
Pain Relief Outcomes
Mean (± SEM) baseline (n = 22) overall back or leg pain scores (VNRS) were 8.4 ± 0.2 (Fig. 2). At 3 months (n = 12), average VNRS overall back or leg pain scores decreased to 1.9 ± 0.5, at 6 months (n = 16) to 2.6 ± 0.5, and at 12 months (n = 20) to 2.0 ± 0.5 (Fig. 2). Analysis using Friedman’s ANOVA revealed a significant change throughout all visits (Friedman test: χ23 = 20.450, p < 0.0005, n = 8). Post hoc analyses (Wilcoxon signed rank) demonstrated a significant improvement at all timepoints compared to baseline (Wilcoxon test: baseline to 3 months: p < 0.005, n = 12; baseline to 6 months: p < 0.0005, n = 16; baseline to 12 months: p < 0.0001, n = 20).
Patient-reported outcomes. Mean (± SEM) baseline (n = 22) overall back or leg pain scores (VNRS) were 8.4 ± 0.2. After 3 months (n = 12), average VNRS overall back or leg pain scores decreased to 1.9 ± 0.5, at 6 months (n = 16) to 2.6 ± 0.5, and at 12 months (n = 20) to 2.0 ± 0.5. Analysis using Wilcoxon signed rank revealed a significant improvement at all timepoints compared to baseline
At 3 months, there were 91.7% (n = 11 of 12 patients) responders (≥ 50.0% pain relief), and 50.0% (n = 6 of 12 patients) high-responders (≥ 80.0% pain relief). At 12 months, 90.0% (n = 18 of 20 patients) of patients were responders, and 60.0% (n = 12 of 20) of patients were high-responders (Fig. 3). Responder and high-responder rates observed at 3 months and 12 months in this real-world cohort were consistent and no difference could be observed with both the AVALON [3 months: 80.0% (n = 36 out 45 patients); 42.2%; (n = 19 out 45 patients); 12 months: 81.4% ( n = 35 out 43 patients), 53.5%; (n = 23 out 43 patients)] and EVOKE [3 months: 87.9% (n = 51 out 58 patients); 62.1% (n = 36 out 58 patients); 12 months: 89.1% (n = 49 out 55 patients), 60.0% (n = 33 out 55 patients)] studies (Fig. 3). The Mann–Whitney U test results revealed no difference between the real-word data (AZN) and the AVALON and the EVOKE responder and high-responder rates (%).
Responder and high-responder rates. The vertical axis indicates (high)-responder rates in %. Patients who showed exceptional pain relief were defined as high-responders (≥ 80.0%) and patients who responded with ≥ 50.0% pain relief were defined as responders. At 12 months, there were 90.0% responders and 60.0% high-responders. There were no significant differences at 3 months and 12 months between the real-world data release (AZN) and the AVALON (3 months: 80.0%; 42.2%; 12 months: 81.4%, 53.5%) and the EVOKE (3 months: 87.9%; 62.1%; 12 months: 89.1%, 60.0%) studies
Percent pain relief observed at 3 months and 12 months in this real-world cohort [77.2% (n = 12); 76.8% (n = 20)] was consistent and therefore no significant differences with both the AVALON [71.2% (n = 45); 73.6% (n = 43)] and EVOKE [78.1% (n = 58); 76.7% (n = 55)] studies were observed (Fig. 4A.). The Mann–Whitney-U test results revealed no difference between the real-word data and the AVALON and the EVOKE pain relief (%) results.
Patient-reported outcomes. A Comparison of overall pain relief (%) to the AVALON12 and EVOKE3 studies. No significant differences at 3 months and 12 months between the real-world data release (77.2%; 76.8%) and the AVALON (71.2%; 73.6%) and EVOKE (78.1%; 76.7%) studies. B Patient satisfaction was reported; after 12 months, 85.0% of patients were very satisfied, quite satisfied, or satisfied
Patient Reported Satisfaction Outcomes
During standard of care follow-up visits, patient satisfaction was collected (Fig. 4B). At the 3-month, 6-month, and 12-month visits, 100.0% (n = 12), 93.8% (n = 16), and 85.0% (n = 20) of the patients, respectively, reported being very satisfied, satisfied, or quite satisfied with the ECAP-controlled closed-loop therapy.
Recording and Characterizing ECAPs
ECAPs could be recorded in all patients (Fig. 5). In total, approximately 19.9 billion ECAPS were recorded from 20 patients during the 12-month period. As an example, 865,236,385 (0.86 billion) ECAPs were recorded from one particular patient during the course of 1 year.
Electrophysiological data, device parameters, and usage were analyzed to get a better understanding of the prescribed therapy. Data for 1 week prior to the patient’s 3-month, 6-month, and 12-month visits were used for this analysis. Median values across the study cohort at 3 months, 6 months, and 12 months are shown for the in-clinic maximum (Fig. 6). Actual out-of-clinic activation is summarized here by the mode ECAP amplitude (dot; Fig. 6). If the activation plot was not analyzable due to technical issues or were not captured, the values were not taken into consideration. Therefore, not all patients have activation plot data presented here.
Median (IQR: Q1–Q3) data at 3 months (n = 11), 6 months (n = 14), and 12 months (n = 15) revealed the ECAP amplitude at perception threshold to be 1.0 µV (− 0.3 to 2.4) at 3 months, − 0.8 µV (− 1.5 to 3.8) at 6 months, and -0.6 µV (− 1.3 to 1.1) at 12 months. The ECAP amplitude at the maximum discomfort level was 49.3 µV (28.3–83.9) at 3 months, 48.0 µV (29.4–74.7) at 6 months, and 45.8 µV (26.9–76.9) at 12 months. The mode ECAP amplitude target, the patients’ actual activation outside the clinic, was at every timepoint greater than the median perception threshold at each visit interval at 7.9 µV (4.5–20.9) at 3 months, 16.5 µV (1.9–20.6) at 6 months, and 11.7 µV (0.4–31.8) at 12 months.
At 12 months, the patients (n = 20) used their device 93.5% (median; IQR: Q1–Q3: 68.6–97.4%) of the time. The median stimulation frequency was 30.0 Hz (30.0–30.0) and median pulse width was 300.0 µs (237.5–350.0; n = 20). Patients used their patient controller to adjust stimulation or program once (median) every three days, i.e., 0.4 (0.3–1.0) adjustments per day (n = 20).
The mean (± SEM) CV for antidromic (n = 10) and orthodromic (n = 8) ECAP recordings were 58.4 m/s (± 2.3) and 55.3 m/s (± 1.3), within the range of Aβ-fibers [20, 21].
Recording and characterizing ECAPs. Example of an individual activation plot from an individual patient. A Recording and measurement of ECAPs from the patient in-clinic; perception threshold was 8.4 mA, comfort range was 9.4–12.4 mA, and maximum (which is the highest level of stimulation the patient could tolerate for 1 min) was 13.8 mA. The recorded neural signal consisted of a positive P1 peak followed by a negative N1 peak and a second positive P2 peak. The ECAP amplitude (µV) grew as current increased. Data have been extracted from the ‘Clinical Data Viewer’ software embedded in the clinical programmer for the system and plotted in Excel. B The same example of the activation plot from A. captured as a screenshot from the programming application for the closed-loop SCS system. The activation plot data display the relationship between current amplitude (mA) and the corresponding neural response
In-clinic and out-of-clinic spinal cord activation. From the bottom, first is the ECAP perception threshold amplitude [3 months: 1.0 µV (n = 11); 6 months: − 0.8 µV (n = 14); 12 months: − 0.6 µV (n = 15)]. Patients used their closed-loop SCS at 12 months above the ECAP threshold (mode: 3 months: 7.9 µV; 6 months: 16.5 µV; 12 months: 11.7 µV). *If the activation plot was not analyzable due to technical issues or data were not captured, the values were not taken into consideration. Therefore, not all patients have activation plot data presented here
Discussion
This prospective, single-center, data collection study in the Netherlands provides the first report of the ‘real-world’ clinical outcomes and the electrophysiological and device data for the treatment of chronic pain of the back or leg, related to PSPS type 2, using a novel ECAP-controlled closed-loop SCS System.
Outcome data from these clinical experiences reveal sustained pain relief over a 12-month period, with a substantial proportion of patients experiencing more than 80% pain relief, complemented by high levels of patient satisfaction. These outcomes parallel those observed in the AVALON prospective, multicenter, single-arm study [12] and the EVOKE double-blind, multicenter, randomized, controlled trial [3]. Not all randomized controlled trials in SCS have been followed up with similar results from real-world cohorts. The data presented in this study are promising and encourage further investigations of this system to confirm its effectiveness in real-world settings through larger, multi-center studies, as the major weakness of this work is the small sample size taken from a single center’s experience.
The ECAP-controlled, closed-loop SCS system used in this study automatically adjusts the stimulation output on every stimulus to consistently activate the same volume of axons determined to be therapeutic without causing overstimulation-related side-effects. Each patient’s response to stimulation is assessed by way of an ‘Activation Profile’, as shown in Fig. 5, and previously described [19]. Traditional, open-loop SCS systems deliver a fixed stimulation output without accounting for normal physical and physiological processes [8, 22]. In systems that evoke stimulation-induced sensation, often referred to as paresthesia, these processes can cause fluctuations in sensation intensity which is believed to limit the efficacy of SCS therapy. To address this problem, an SCS system with a 3-axis accelerometer was developed a decade ago which can detect gross postural changes (e.g., it can differentiate between lying supine vs. walking but not sitting vs. standing), and be programmed to switch between preset open-loop stimulation settings in response, so a type of feed-forward control system [22, 23] (p 201). In a randomized controlled crossover study of this feature, 86.5% of patients in the accelerometer-enabled group (A-ON) reported "improved pain relief with no loss of convenience, or improved convenience with no loss of pain relief", compared to the accelerometer off group (the control A-OFF group). A key objective measure to support these findings was the number of ‘button presses’ on the patient’s remote control to change stimulation amplitude. Patients in the A-ON group had 41% fewer interactions with their remote control as compared to the A-OFF group, on average 18.2 button presses per day (standard deviation: SD 38.9) as compared to 30.7 per day (SD 63.5) [22]. There was no difference in actual pain relief between groups, and unfortunately neither group of patients achieved a clinically meaningful decrease in pain intensity from baseline. The A-ON group reported a decrease of 1.8 points on an 11-point Numerical Pain Rating Scale (NPRS 0–10), while the A-OFF group reported a decrease of 1.5 points [22]. A minimal clinically important improvement in pain intensity is well accepted as being at least a 2-point reduction on an 11-point NPRS [24]. Despite the lack of effect on pain intensity, the introduction of this system, and this study, produces an objective measure for the patient experience with the system, defined as button presses. SCS studies generally suffer from a lack of objectivity in their outcome measures and patient reports, as pain is an inherently subjective experience. In our study, the median number of button presses per day was 0.4, suggesting that patients utilizing ECAP-controlled, closed-loop SCS rarely need to interact with their therapy, and significantly less so compared to previously reported data from the feed-forward system used in the study by Shultz and colleagues [22]. However, it could also be the case that patients in the Shultz et al. study were interacting with their system more often as they sought better pain relief, whereas, in our study group, clinically important pain relief was attained in the first 4 weeks and maintained through 1 year, so perhaps had less reason to interact with their system [22]. To our knowledge, there are no data reported regarding the frequency of patient–device interactions for SCS systems or stimulation settings which do not elicit sensations. While it could be assumed that such interactions would be very few, the field would benefit from this type of objective data reporting in all studies on SCS systems to allow comparisons between different systems and waveforms. A recommendation for increased transparency in SCS settings and usage for studies of SCS in chronic pain was recently published by Katz and colleagues [13].
One of the most attractive aspects of this new system is the type and amount of objective data that the device can capture. Figure 6 describes the average perception threshold for the study cohort and the mode ECAP amplitude for the group at 3 months, 6 months, and 12 months. These data represent a performance measure for the ECAP-controlled system, have been reported previously in the first-in-human and pivotal studies [3, 10,11,12] for this system, and are replicated here. It has been postulated that consistent control of spinal cord activation leads to better patient outcomes in terms of pain relief [3, 11, 19]. We observed that the system made intended output adjustments at the same frequency as the programmed rate to achieve spinal activation within the prescribed range, and that patients in our study also experienced significant pain relief. Additionally, the patients used the device in median 93.5% of the time, which means that they ran the stimulation day and night. However, further research is required to convincingly confirm this hypothesis.
Additional data taken from the device included the CV of the stimulated fibers, which was in line with previous recordings from this system in earlier studies and those anticipated for Aβ-fiber activation [21, 25,26,27,28,29,30]. It is unknown at this time as to whether or not this information may be helpful in predicting outcomes for SCS patients or even monitoring the health of the dorsal columns, but this is not something we will ever determine if we do not use this system to its full potential and capture this data.
Limitations of this study include a small sample size, single-center results, no blinding, and no control arm, missing data due to pandemic-related visit cancellations, and some additional missing data due to time constraints in clinic. All of which limit the generalizability of the presented findings. Additionally, our study would have benefited from assessments of further domains which can be affected by chronic pain, such as quality of life and function to support our findings. A more systematic approach to device data collection, particularly in the acquisition of activation plots, should be considered in future work. While there are significant missing data at 3 months and 6 months, all the implanted patients were seen at 12 months, so we do not believe that the earlier missing data points affect the conclusions.
Conclusion
The results of this data collection study provide novel insights into the beneficial effect of ECAP-controlled, closed-loop SCS in a real-world setting. The results we present demonstrate a noteworthy maintenance of pain relief over a 12-month period, and corroborate the outcomes observed in the AVALON prospective, multicenter single-arm study [12] and the EVOKE double-blind, multicenter, randomized, controlled trial [3, 11] previously published. These initial ‘real-world’ data on ECAP-controlled, closed-loop SCS in a real-world clinical setting appear to be promising.
References
Shealy CN, Taslitz N, Mortimer JT, Becker DP. Electrical inhibition of pain: experimental evaluation. Anesth Analg. 1967;46(3):299–305.
Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–88.
Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–34. https://doi.org/10.1016/S1474-4422(19)30414-4.
Duarte RV, Soliday N, Leitner A, Taylor RS. Health-related quality of life associated with pain health states in spinal cord stimulation for chronic neuropathic pain. Neuromodul Technol Neural Interface. 2020. https://doi.org/10.1111/ner.13267.
Duarte RV, Nevitt S, Maden M, et al. Spinal cord stimulation for the management of painful diabetic neuropathy: a systematic review and meta-analysis of individual patient and aggregate data. Pain. 2021;162(11):2635–43. https://doi.org/10.1097/j.pain.0000000000002262.
Eldabe S, Thomson S, Duarte R, et al. The effectiveness and cost-effectiveness of spinal cord stimulation for refractory angina (RASCAL Study): a pilot randomized controlled trial: SCS for refractory angina (RASCAL Study). Neuromodul TechnolNeural Interface. 2016;19(1):60–70. https://doi.org/10.1111/ner.12349.
Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687–98.
Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153(3):593–601. https://doi.org/10.1016/j.pain.2011.11.023.
Parker JL, Karantonis DM, Single PS, et al. Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation. 2013;16(4):295–303. https://doi.org/10.1111/ner.12053. (discussion 303).
Brooker C, Russo M, Cousins MJ, et al. ECAP-controlled closed-loop spinal cord stimulation efficacy and opioid reduction over 24-month: final results of the prospective, multicenter, open-label Avalon study. Pain Pract. 2021. https://doi.org/10.1111/papr.13008.
Mekhail N, Levy RM, Deer TR, et al. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurol. 2022;79(3):251–60. https://doi.org/10.1001/jamaneurol.2021.4998.
Russo M, Brooker C, Cousins MJ, et al. Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label Avalon study. Neurosurgery. 2020. https://doi.org/10.1093/neuros/nyaa003.
Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain. 2021;162(7):1935–56. https://doi.org/10.1097/j.pain.0000000000002204.
Schwartz JL. Real-world evidence, public participation, and the FDA. Hastings Cent Rep. 2017;47(6):7–8.
De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401–21. https://doi.org/10.1093/pm/pnx241.
Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66. https://doi.org/10.1111/ner.12698.
Kapural L, Yu C, Doust MW, et al. Novel 10-khz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–60. https://doi.org/10.1097/ALN.0000000000000774.
Deer TR, Russo MA, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): Recommendations for Surgical Technique for Spinal Cord Stimulation. Neuromodul Technol Neural Interface. 2022;25(1):1–34.
Parker J, Karantonis D, Single P. Hypothesis for the mechanism of action of ECAP-controlled closed-loop systems for spinal cord stimulation. Healthc Technol Lett. 2020;7(3):76–80. https://doi.org/10.1049/htl.2019.0110.
Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of neural science. 5th ed. McGraw Hill Professional; 2013.
Smith ESTJ, Lewin GR. Nociceptors: a phylogenetic view. J Comp Physiol A. 2009;195(12):1089–106. https://doi.org/10.1007/s00359-009-0482-z.
Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician. 2012;15(1):1–12.
Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11(3):553–63. https://doi.org/10.1007/s13311-014-0280-3.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. https://doi.org/10.1016/j.jpain.2007.09.005.
Glees P, Soler J. Fibre content of the posterior column and synaptic connections of nucleus gracilis. Z Zellforsch Mikrosk Anat. 1951;36(4):381–400.
Jones SJ, Edgar MA, Ransford AO. Sensory nerve conduction in the human spinal cord: epidural recordings made during scoliosis surgery. J Neurol Neurosurg Psychiatry. 1982;45(5):446–51. https://doi.org/10.1136/jnnp.45.5.446.
Magladery J, Porter W, Park A, Teasdall R. Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp. 1951;88(6):499–519.
Shimizu H, Shimoji K, Maruyama Y, Sato Y, Harayama H, Tsubaki T. Slow cord dorsum potentials elicited by descending volleys in man. J Neurol Neurosurg Psychiatry. 1979;42(3):242–6. https://doi.org/10.1136/jnnp.42.3.242.
Shimoji K, Kano T, Higashi H, Morioka T, Henschel EO. Evoked spinal electrograms recorded from epidural space in man. J Appl Physiol. 1972;33(4):468–71. https://doi.org/10.1152/jappl.1972.33.4.468.
Tsuyama N, Tsuzuki N, Kurokawa T, Imai T. Clinical application of spinal cord action potential measurement. Int Orthop. 1978. https://doi.org/10.1007/BF00266001.
Acknowledgements
The study was sponsored by Saluda Medical. The authors gratefully thank the participants of the study and acknowledge the Antonius Hospital Unit for their support and assistance in this work and would also like to thank Ralph Aarsman, Gerry Aikema, Joris Brouns and Maeve Willen for their support during data collection and analysis.
Funding Information
This study was sponsored and funded by Saluda Medical. The sponsor funded the publication review and the Rapid Service Fee. We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
Author Contributions
Harold Nijhuis conducted the study including data collection and analysis, conducted statistical analysis and interpretation of the data, and wrote the manuscript; Frank Huygen interpreted the data and reviewed and edited the manuscript; Willem-Jan Hofsté conducted the study including data collection and reviewed and edited the manuscript; Imre P. Krabbebos conducted the study including data collection and reviewed and edited the manuscript; Dave Mugan designed the study, interpreted the data and wrote the manuscript; Birte E. Dietz performed data and statistical analysis, interpretation of the data, and wrote the manuscript; All authors approved the final manuscript and agree with its submission.
Disclosures
Harold Nijhuis is a paid consultant for Saluda Medical, Abbott, and Salvia. Harold Nijhuis has a research grant with Abbott. Frank Huygen reports personal fees from Abbott, grants, personal fees from Saluda, personal fees from Boston Scientific, personal fees from Grunenthal, personal fees from Pfizer, outside the submitted work. Willem-Jan Hofsté is a paid consultant for Saluda Medical and Abbott. Birte E. Dietz and Dave Mugan are employed by Saluda Medical. There are no other relationships that might lead to a conflict of interest in the current study.
Compliance with Ethics Guidelines
This observational data collection adhered to the ethical principles of the Declaration of Helsinki. In the Netherlands, a prospective data collection without any intervention does not fall under the Medical Research Involving Human Subject Act (WMO). This means that no medical ethical committee approval is required for this type of study in the Netherlands, as confirmed by the ethical committee (Amsterdam University Medical Centers, W17_309#17.362).
Data Availability Statement
The data was available for review by all the contributing authors. All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nijhuis, H.J.A., Hofsté, WJ., Krabbenbos, I.P. et al. First Report on Real-World Outcomes with Evoked Compound Action Potential (ECAP)-Controlled Closed-Loop Spinal Cord Stimulation for Treatment of Chronic Pain. Pain Ther 12, 1221–1233 (2023). https://doi.org/10.1007/s40122-023-00540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00540-y