Abstract
Chronic pain, a complex and debilitating condition, involves intricate interactions between central and peripheral inflammatory processes. Cytokines, specifically tumor necrosis factor (TNF) and interleukins (IL), are key mediators in the initiation and maintenance of chronic pain states. Sensory neurons expressing receptors for cytokines like TNF, IL-1, and IL-6 are implicated in peripheral sensitization, contributing to increased signaling of painful sensations. The potential of targeting TNF and IL for therapeutic intervention in chronic pain states is the focus of this review, with preclinical and clinical evidence supporting the use of TNF and IL modulators for pain management. The physiological and pathological roles of TNF in neuropathic pain is complex. Experimental evidence highlights the effectiveness of TNF modulation in mitigating pain symptoms in animal models and displays promising outcomes of clinical trials with TNF inhibitors, such as infliximab and etanercept. ILs, a diverse group of cytokines, including IL-1, IL-6, and IL-17, are discussed for their contributions to chronic pain through inflammation and peripheral sensitization. Specific IL modulators, such as secukinumab and tocilizumab, have shown potential in managing chronic neuropathic pain, as demonstrated in various studies and clinical trials. The pharmacokinetics, safety profiles, and challenges associated with TNF and IL modulators highlight the need for cautious medication monitoring in clinical practice. Comparative evaluations have revealed distinct efficacy and safety profiles among different cytokine modulators, emphasizing the need for personalized approaches based on the specific underlying causes of pain. Further research is necessary to elucidate the intricate mechanisms by which cytokines contribute to chronic pain, as well as to understand why they may affect pain differently in various contexts. Additionally, long-term safety profiles of cytokine modulators require more thorough investigation. This continued exploration holds the promise of enhancing our comprehension of cytokine modulation in chronic pain and shaping more potent therapeutic strategies for the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic pain involves complex interactions of pro-inflammatory cytokines, particularly tumor necrosis factor (TNF) and interleukins (IL), contributing to increased pain sensation in the peripheral and central nervous systems. |
TNF and IL modulators show promise in treating chronic pain states, offering alternative options for treatment beyond traditional approaches. |
The use of TNF and IL modulators presents challenges, including potential safety concerns. Vigilant monitoring and personalized approaches are necessary in clinical practice. |
Ongoing research focuses on exploring long-term safety and evaluating the potential of combination therapies to deepen our understanding and improve therapeutic strategies in management of chronic pain states. |
Introduction
Pathologic pain states encompass a range of chronic conditions characterized by persistent, often debilitating pain, where central and peripheral inflammatory processes play a pivotal role. Cytokines, which are secreted proteins that act on other cells via autocrine, paracrine, or endocrine pathways, can be anti-inflammatory or pro-inflammatory. Although many cell types can produce cytokines, the primary mediators are macrophages and helper T-cells. Macrophages are responsible for the secretion of pro-inflammatory cytokines. Pro-inflammatory cytokines, including the families of tumor necrosis factor (TNF) and interleukins (IL), are key mediators in the pathophysiology of chronic pain, contributing to the initial sensitization and maintenance of pain states. Specifically, TNF-α, IL-1β, IL-6, and IL-17 have been attributed to the pathology behind pain [1]. Additionally, increased levels of cytokines such as TGF-B and CCL19 have been measured in various neurologic pain pathologies [2]. Conversely, TGF-β1, IL-4, IL-10, IL-11, and IL-13 are key players in ant-inflammatory states because they can dampen the pro-inflammatory pathway [1].
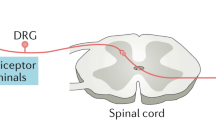
Sensory neurons have receptors for TNF, IL-1, and IL-6, as well as many other pro-inflammatory mediators [3]. Research has found that activation of these cytokine receptors on peripheral nociceptors of the dorsal root ganglia occurs in response to pain, bolstering the use of their inhibition as a potential treatment for pain management [4]. This peripheral sensitization can enhance excitatory signaling from nociceptors in the dorsal horn of the spinal cord, with subsequent sensitization of secondary sensory neurons that elicit increased duration of allodynic and hyperalgesic responses. This neuroplastic process, called central sensitization, can also extend the receptive field for stimuli beyond the injured tissue and facilitate the spread of pain (secondary hyperalgesia) [3]. Activated glial cells in the central nervous system can secrete pro-inflammatory cytokines, contributing to neuropathic pain. In the peripheral nervous system, non-neuronal cells have the capability to reinforce the pro-inflammatory pathway involved in chronic and acute pain states. Peripheral and central sensitization can occur synergistically or independently of one another, but both have major roles in the maintenance of chronic pain states, making them a focus of potential therapeutic strategies. Creating drugs that target TNF and IL can provide patients experiencing chronic pain with an alternative medication regimen to those historically used, such as opioids [1]. This review aims to better understand the intricate roles that TNF and IL have in chronic pain pathways with a special focus on the specific cytokines that offer the most promising avenues for targeted therapeutic interventions.
Tumor Necrosis Factor
TNF is a pro-inflammatory cytokine that plays a role physiologically and pathologically [5, 6]. TNF is a 26-kDa 233-amino acid transmembrane protein expressed on cell surfaces that is produced mainly by macrophages, and by T and B lymphocytes, natural killer cells, mast cells, neutrophils, fibroblasts, and osteoclasts [5]. There is a family of TNF, including alpha (α) and beta (β) subtypes, among others. TNF also regulates embryo development, the sleep–wake cycle, and lymph node formation. Its inflammatory mechanism acts as part of the host defense against bacterial and viral infections, but, with mutations and alterations, TNF can also play a role in autoimmune diseases. As part of the host defense system, TNF can act throughout the body, for example, the nervous and gastrointestinal systems, to induce necrosis and apoptosis, which lead to cell death. The necrotic pathway involves cell swelling, organelle destruction, and eventual cell lysis, while the apoptotic pathway involves cell shrinking, condensed body formation, and final DNA fragmentation [6]. There are two receptors that TNF binds to, TNFR1 and TNFR2; TNFR1 is expressed on all human tissue cells, while TNFR2 is expressed in immune cells, neurons, and endothelial cells [5]. Once TNF binds to these receptors, a signaling cascade is triggered that initiates the pathway to activate nuclear factor-κB (NF-κB), activating protein-1 (AP-1), and mixed lineage kinase domain-like (MLK-L), which are transcription factors that initiate inflammatory and cell destruction pathways [6]. Inflammation and cell destruction pathways initiated by TNF are implicated in pain.
Experimental evidence demonstrates the involvement of TNF in amplifying nociceptive signaling, promoting neuroinflammation, and inducing hyperalgesia in various pain models. Neuropathic pain is nerve pain that has the potential to lead to hypersensitivity to stimuli, abnormal sensations, and nociceptive responses to stimuli that would otherwise not be painful; it often presents after an injury. The origin of neuropathic pain is unclear, but it appears that there is both a central and peripheral role at play. There is evidence of pro-inflammatory mediators in both mechanisms of pain. Neuropathic pain is of importance to investigate due to its complexity in treatment as well as impairment of quality of life for those who experience it. Post-injury inflammation includes mast cell degranulation and recruitment of macrophages and neutrophils; this inflammatory environment is believed to be pivotal to the development of neuropathic pain. TNF-α is thought to be a part of this inflammatory environment [7]. Evidence in animal models shows that TNF-α is found at the site of injury and is still up-regulated post-injury [8, 9]. Additionally, the TNF receptors, TNFR1 and TNFR2, have been shown to be locally unregulated in injured neurons that are the source of neuropathic pain [10]. Upon animal model testing, injection of TNF-α in rats has been shown to reproduce the pain hypersensitivity symptom that neuropathic pain induces [11, 12]. In human studies, nerve biopsies have been obtained from patients experiencing painful neuropathy, and these biopsies also illustrate increased TNF-α expression (13).
Preclinical studies have explored modulation of TNF for pain management, and efficacy has been shown in attenuating pain behaviors in animal models. The structure of the TNF ligand and its receptor can be modulated to prevent signaling and further phosphorylation and expression along the pain pathway. The transmembrane portion of the TNF molecule can act as a receptor to be down-regulated via reverse signaling [7]. This “gene therapy” has been trialed to reduce pain responses. In rat models with injured nerves, a fusion protein that modulates the TNF ligand was developed to mitigate the levels of TNF-α [14]. In animal models with neuropathic pain, local or spinal administration of TNF-α antagonists have also attenuated pain behaviors [15, 16]. Clinical trials evaluating the use of TNF inhibitors in conditions like neuropathic pain have demonstrated promising outcomes in pain management. Two specific drugs already on the market, infliximab and etanercept, have also shown progress in models for pain control. Systemic treatment with TNF-α inhibition has not been proven to be as beneficial in follow-up trials, but further investigation is necessary since there have been promising results with other mechanisms of delivery [17, 18].
Interleukin (IL) Modulators
ILs are a diverse group of cytokines participating in the complex intercellular communication displayed in immune and inflammatory responses. Numerous cell types can secrete and bind to cell surface receptors in autocrine and paracrine fashions [19]. IL can then exert pro-inflammatory or anti-inflammatory effects by promoting activation and migration of immune cells, as well as cause direct damage to the nervous system [19, 20].
IL-6 and IL-17, among others, are pro-inflammatory cytokines strongly associated with the pathogenesis of chronic pain states through inflammation and the process of peripheral sensitization [21]. These IL and other inflammatory mediators bind to receptors on peripheral primary afferent nociceptors and change the ion channels of the fibers, reducing their stimulation threshold and amplifying fiber sensitivity. They also facilitate recruitment of additional fibers to the injury site, increasing nociceptive input. These combined effects manifest as the perception of innocuous stimuli as painful (allodynia) and a heightened response to pain (hyperalgesia) [22].
The IL-17 family of cytokines consists of IL-17A through IL-17F; however, IL-17A is typically referred to solely as IL-17. It is primarily released by activated CD4 + Th17 cells and binds to IL-17R, expressed by hematopoietic cells, endothelial cells, epithelial cells, and fibroblasts [23]. Its downstream signaling effects contribute to inflammatory pain by promoting gene expression involving the recruitment of immune cells and secretion of inflammatory cytokines, mainly IL-6. Preclinical studies have shown that IL-17 can also directly contribute to neuroinflammation and chronic neuropathic pain via direct neuronal sensitization [24]. IL-17 knockout mice were shown to have decreased mechanical hypersensitivity and neuroinflammatory response following a partial sciatic nerve ligation in comparison to controls, demonstrated by significantly fewer T-cells and macrophages at the site of nerve injury and in the dorsal root ganglia of the mice. Furthermore, local IL-17 injections in uninjured mice produced hyperalgesia and recruitment of immune cells to the injection site [23]. Another study showed enhanced nociceptor sensitization to mechanical stimuli following IL-17 injection into mice knee joints, but the sensitization persisted even with neutralization of inflammatory mediators TNF-α and IL-6. The cell bodies of the primary sensory neurons located in the dorsal root ganglia of these mice were shown to express IL-17R, demonstrating that IL-17 alone can contribute to mechanisms involved in chronic pain, even when some of its inflammatory effects are down-regulated [25]. These data support the emerging IL-17 and IL-17R targets in the potential treatment of inflammatory disease and chronic neuropathic pain.
Secukinumab, a human monoclonal antibody against IL-17A, is currently indicated in the treatment of psoriatic arthritis, ankylosing spondylosis, and rheumatoid arthritis [24]. Multiple sclerosis (MS) has strong associations with IL-17 in its pathogenesis. One study showed that treatment with secukinumab may reduce brain lesion activity seen on MRI in patients with MS [26]. Ixekizumab is another humanized IL-17A neutralizing antibody that treats psoriasis and psoriatic arthritis. While there are no direct studies on these IL-17 antibody drugs for the treatment of chronic pain alone, they have been shown to decrease pain symptoms in patients overall. Brodalumab is a human monoclonal antibody against IL-17RA used to treat psoriasis and rheumatoid arthritis, and evidence has suggested that brodalumab may be useful for neuropathic pain [24]. In mice treated with paclitaxel, a chemotherapy agent, mouse IL-17RA antibodies were shown to reduce hyperexcitability in chemotherapy-induced peripheral neuropathy [24, 27]. Further studies are needed to determine the potential for IL-17 and IL-17RA blockers in the treatment of chronic pain.
IL-6 is produced by T- and B-lymphocytes, macrophages, and fibroblasts in response to inflammatory stimuli like IL-1, IL-17, and TNF-α. It promotes T- and B-cell differentiation and induces acute phase protein production. Its receptor, IL-6R, can be both in a membrane-bound or soluble form, and it has a gp130 signaling component that is expressed on all cell membranes [20, 28]. Thus, IL-6 has pleiotropic effects that characterize it as a key cytokine in the pathogenesis of numerous conditions like rheumatoid arthritis, multiple sclerosis, cancer pain, and neuropathic pain [20]. IL-6 shows similarities to IL-17 in that it is implicated in both inflammatory pain and neuronal sensitization. In rat models of a sciatic nerve injury, a positive correlation was shown between the number of IL-6 positive cells and the degree of mechanical allodynia [20, 29]. Another study demonstrated central sensitization that was induced by IL-6. Mice knee joints and spinal cords were treated with IL-6 and soluble IL-6R, resulting in hyperresponsiveness by the dorsal horn neurons upon mechanical stimulation of the mice knee or ankle joints [20, 30]. IL-6 and IL-6R levels have also been shown to be increased in mice models with intervertebral disc injury. Following injection of an IL-6 inhibitor, the mice showed decreased levels of calcitonin gene-related peptide, a pain-related protein, in their dorsal root ganglia. This provides evidence that targeting IL-6 may ameliorate chronic pain symptoms [20, 31].
Mechanisms of Action and Pharmacology
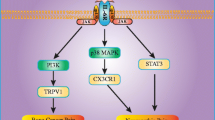
Both TNF and IL modulators exert their effects by disrupting cytokine signaling, dampening neuroinflammation, and modulating synaptic transmission in pain pathways. TNF inhibitors block the activation of the NF-kB transcription factor, thus blocking its downstream response which releases macrophages, T-cells, B-cells, and other proinflammatory cytokines including IL-1 and IL-6 [32]. The IL modulators referenced in managing various pain types include both IL-1 and IL-6 receptor antagonists. These block many facets of the inflammatory response, including macrophage stimulation, lymphocyte activation and differentiation, and fever, and the release of acute phase reactants [19]. While these drug classes act on different parts of the pain pathway, both work to limit the inflammatory response.
The pharmacokinetics of specific TNF inhibitors and IL modulators are summarized in Tables 1 and 2. While the half-life (t½) is provided in addition to onset and duration of action, these times can be dependent on both the individual and their condition. Therefore, the efficacy and utility of these values is unreliable in pain management and will be case-specific [33].
Some of the most common adverse effects of specific TNF inhibitors and IL modulators are listed in Tables 1 and 2. Each of the TNF-α inhibitors referenced in Table 1 has been given a black-box warning by the FDA noting the dangers of infection risk for patients. As for IL modulators, referenced in Table 2, sarilumab and tocilizumab both have black box warnings for risk of serious infection. While anakinra does not have a black box warning for infection, it is associated with infection as an adverse effect [34]. It is imperative to underscore the need to carefully consider the risks associated with long-term cytokine modulation. Patients should be screened for tuberculosis and hepatitis B and C out of concern for reactivation of these infections. These modulators should be withheld if there is any sign of infection [32].
Comparative Analysis and Future Perspectives
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Comparative evaluations of TNF and IL modulators reveal differential efficacy and safety profiles, suggesting personalized approaches based on specific pain etiologies. The efficacy of TNF and IL modulators for treatment of pain has shown mixed results based on the etiology of the pain. Both TNF-α inhibitors and anti-IL-6R antibodies have been studied for pain associated with sciatica with success. The TNF-α inhibitor, infliximab, was given to patients for severe sciatica. Patients were treated with a 3-mg/kg IV infusion, and patients reported a significantly higher percentage of painlessness compared to the control group 1 year after infusion [17]. Cases with sciatica caused by lumbar spinal stenosis were treated with the anti-IL-6 receptor antibody, trocilizumab, via epidural administration; resulting visual analogue scale (VAS) pain scores in the leg and lower back were significantly lower in the tocilizumab group than in the control group [42]. Similar results were seen when studying the effects of TNF and IL modulators on pain related to rheumatoid arthritis. Certolizumab (anti-TNF) and sarilumab (IL-6 inhibitor) both showed significant decreases in rheumatoid arthritis-associated pain when compared to controls, but both had significant increases in rates of infection [43, 44]. However, when studied in patients with osteoarthritis in the hand, humira (adalimumab), a TNF-α inhibitor showed no significant difference in VAS pain scores with the placebo [45]. Similarly, AMG 108, an antibody to the IL-1R, showed no change in WOMAC pain scores compared to the placebo in knee osteoarthritis patients [46].
Challenges in targeting TNF and IL signaling pathways, including incomplete understanding of their complex interactions and potential compensatory mechanisms, require further investigation. Clinical trials face challenges in establishing the efficacy of TNF-α inhibitors and IL modulators due to varying outcomes across different pain etiologies. Understanding these differences is crucial for developing effective treatments. TNF-α inhibitors seem to be effective in only some types of neuropathic pain, as positive results were seen in mouse models with diabetic neuropathy, but not in pain related to disc herniation [47]. Currently, the mechanisms by which the cytokines treat pain is not fully understood, making it difficult to effectively treat patients. IL-17 is a target for neuropathic pain, but some studies show that IL-17 plays a role in later stages of nerve damage. At the same time, some IL-17 blockers, such as secukinumab, were effective at rapidly treating neuropathic pain [24]. These variations in the results are complicated by the multifactorial pain and inflammation pathway, where the effects of individual cytokines may not be enough to treat complex chronic pain etiologies [48].
Emerging strategies, such as combination therapies or novel targets within cytokine pathways, hold promise for enhanced pain relief and improved patient outcomes. Combination therapy with a cytokine modulator and another drug class or the use of platelet-rich plasma (due to the cytokines released by plasma having effects on multiple parts of the pain pathway) have shown promising results in the treatment of chronic pain (Table 3) [48].
Discussion
Pathologic pain states are complex conditions characterized by persistent and debilitating pain, where the interplay of central and peripheral inflammatory processes is a vital mediator in this pathology. Sensory neurons expressing TNF, IL-1, and IL-6 receptors are implicated in peripheral sensitization, contributing to enhanced nociceptive signaling and the development of chronic pain. This process extends to central sensitization, where activated glial cells in the central nervous system further contribute to neuropathic pain. Understanding the intricate roles of TNF and IL in chronic pain pathways has prompted research into modulating these cytokines for therapeutic interventions.
TNF, a multifaceted cytokine with several physiologic roles, becomes pathological when dysregulated. Its involvement in neuropathic pain is evident, with increased expression in injured neurons. There is promise for potential applications of TNF-α antagonists in alleviating chronic pain symptoms. Clinical trials with TNF inhibitors like infliximab and etanercept have shown potential for managing neuropathic pain, although these studies have shown varying degrees of success. Through animal models and human testing, TNF plays a role in pathologic pain states.
ILs, a diverse group of cytokines, including IL-6 and IL-17, contribute to chronic pain through inflammation and peripheral sensitization. Targeting IL-17 and IL-6 with specific inhibitors has been shown to be therapeutic in treating certain autoimmune conditions like rheumatoid and psoriatic arthritis, with potential applications in chronic neuropathic pain. For example, tocilizumab and sarilumab are anti-IL-6R monoclonal antibodies that have both demonstrated efficacy for their treatment of rheumatoid arthritis, reducing both inflammation and pain [20]. Furthermore, tocilizumab treatment decreases levels of double stranded DNA antibodies, which may be due to the blockage of IL-6 effects on B cell differentiation, thereby decreasing the levels of autoantibody-producing B cells [49, 50].
Both TNF and IL modulators disrupt cytokine signaling, presenting a potential avenue for pain relief. However, the pharmacokinetics and safety profiles of these modulators vary, requiring careful consideration in patient selection. Due to their mechanisms of action, TNF inhibitors and IL modulators are associated with many potential side effects and safety concerns, including an enhanced susceptibility to infections. The infections may include bacterial, fungal, viral, atypical, or upper respiratory infections. Further investigation on the long-term safety profiles of TNF and IL modulators is essential for establishing their efficacy and safety. Comprehensive studies should address concerns related to infections, immunogenicity, and potential complications associated with long-term use. When it comes to the use and effectiveness of TNF and IL modulators, an individualized approach should be used to prescribe these medications to patients. Considering the complex nature of chronic pain, combination therapies involving cytokine modulators and other pharmacological or non-pharmacological approaches may provide synergistic benefits. Integrating these strategies could optimize pain management outcomes.
Conclusions
Targeting TNF and IL signaling pathways is challenging due to the complex interactions and potential compensatory mechanisms involved. Further investigation is needed to fully understand these pathways and to develop more effective therapeutic strategies. Insights gained from preclinical and clinical studies offer valuable directions for refining therapeutic strategies and advancing the development of targeted cytokine-based interventions for chronic pain. While the focus has been on pro-inflammatory cytokines, additional studies should examine the role of anti-inflammatory cytokines, such as TGF-β1, IL-4, IL-10, IL-11, and IL-13, in chronic pain states. Current research is working towards creating combination therapies and exploring novel targets within cytokine pathways, paving the way for future advancements in pain therapeutics.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Zhang JM, An J. Cytokines, Inflammation and Pain. Int Anesthesiol Clin. 2007;45(2):27–37. https://doi.org/10.1097/AIA.0b013e318034194e.
Pinto LG, Pinho-Ribeiro FA, Verri WA. Editorial: cytokines and pain. front Immunol Internet. 2021. https://doi.org/10.3389/fimmu.2021.788578.
Vanderwall AG, Milligan ED. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol [Internet]. 2019. https://doi.org/10.3389/fimmu.2019.03009.
Crosson T, Roversi K, Balood M, Othman R, Ahmadi M, Wang JC, et al. Profiling of how nociceptor neurons detect danger—new and old foes. J Intern Med. 2019;286(3):268–89. https://doi.org/10.1111/joim.12957.
Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott MF. Tumour necrosis factor signalling in health and disease. F1000Research. 2019;8:F1000 Faculty Rev-111. https://doi.org/10.12688/f1000research.17023.1.
Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328(2):222–5. https://doi.org/10.1016/j.canlet.2012.10.014.
Leung L, Cahill CM. TNF-α and neuropathic pain—a review. J Neuroinflammation. 2010;16(7):27. https://doi.org/10.1186/1742-2094-7-27.
George A, Schmidt C, Weishaupt A, Toyka KV, Sommer C. Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Exp Neurol. 1999;160(1):124–32. https://doi.org/10.1006/exnr.1999.7193.
Shubayev VI, Myers RR. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855(1):83–9. https://doi.org/10.1016/S0006-8993(99)02321-5.
George A, Buehl A, Sommer C. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp Neurol. 2005;192(1):163–6. https://doi.org/10.1016/j.expneurol.2004.11.002.
Wagner R, Myers RR. Endoneurial injection of TNF-α produces neuropathic pain behaviors. NeuroReport. 1996;7(18):2897. https://doi.org/10.1097/00001756-199611250-00018.
Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst JPNS. 2000;5(2):96–100. https://doi.org/10.1046/j.1529-8027.2000.00012.x.
Empl M, Renaud S, Erne B, Fuhr P, Straube A, Schaeren-Wiemers N, et al. TNF-alpha expression in painful and nonpainful neuropathies. Neurology. 2001;56(10):1371–7. https://doi.org/10.1212/wnl.56.10.1371.
Shamji MF, Jing L, Chen J, Hwang P, Ghodsizadeh O, Friedman AH, et al. Treatment of neuroinflammation by soluble tumor necrosis factor receptor Type II fused to a thermally responsive carrier. J Neurosurg Spine. 2008;9(2):221–8. https://doi.org/10.3171/SPI/2008/9/8/221.
Sommer C, Lindenlaub T, Teuteberg P, Schäfers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913(1):86–9. https://doi.org/10.1016/s0006-8993(01)02743-3.
Svensson CI, Schäfers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379(3):209–13. https://doi.org/10.1016/j.neulet.2004.12.064.
Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, et al. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine. 2004;29(19):2115–9. https://doi.org/10.1097/01.brs.0000141179.58778.6c.
Cohen SP, Wenzell D, Hurley RW, Kurihara C, Buckenmaier CC, Griffith S, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107(1):99–105. https://doi.org/10.1097/01.anes.0000267518.20363.0d.
Justiz Vaillant AA, Qurie A. Interleukin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Jan 25]. http://www.ncbi.nlm.nih.gov/books/NBK499840/.
Sebba A. Pain: a review of interleukin-6 and its roles in the pain of rheumatoid arthritis. Open Access Rheumatol Res Rev. 2021;5(13):31–43. https://doi.org/10.2147/OARRR.S291388.
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8–27. https://doi.org/10.1111/imr.12621.
Totsch SK, Sorge RE. Immune system involvement in specific pain conditions. Mol Pain. 2017;1(13):1744806917724559. https://doi.org/10.1177/1744806917724559.
Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain. 2011;12(3):370–83. https://doi.org/10.1016/j.jpain.2010.08.003.
Jiang X, Zhou R, Zhang Y, Zhu T, Li Q, Zhang W. Interleukin-17 as a potential therapeutic target for chronic pain. Front Immunol. 2022;29(13): 999407. https://doi.org/10.3389/fimmu.2022.999407.
Richter F, Natura G, Ebbinghaus M, von Banchet GS, Hensellek S, König C, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64(12):4125–34. https://doi.org/10.1002/art.37695.
Havrdová E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol. 2016;263(7):1287–95. https://doi.org/10.1007/s00415-016-8128-x.
Luo H, Liu HZ, Zhang WW, Matsuda M, Lv N, Chen G, et al. Interleukin-17 regulates neuron-glial communications, synaptic transmission, and neuropathic pain after chemotherapy. Cell Rep. 2019;29(8):2384-2397.e5. https://doi.org/10.1016/j.celrep.2019.10.085.
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. https://doi.org/10.1016/j.jaci.2016.06.033.
Possible role of inflammatory mediators in tactile hypersens...: PAIN [Internet]. [cited 2024 Jan 26]. Available from: https://journals.lww.com/pain/abstract/2000/12010/possible_role_of_inflammatory_mediators_in_tactile.5.aspx DOI: https://doi.org/10.1016/S0304-3959(00)00331-6
Spinal interleukin‐6 is an amplifier of arthritic pain in the rat. [cited 2024 Jan 26]; Available from: https://doi.org/10.1002/art.34384
Sainoh T, Orita S, Miyagi M, Sakuma Y, Yamauchi K, Suzuki M, et al. Interleukin-6 and interleukin-6 receptor expression, localization, and involvement in pain-sensing neuron activation in a mouse intervertebral disc injury model. J Orthop Res. 2015;33(10):1508–14. https://doi.org/10.1002/jor.22925.
Gerriets V, Goyal A, Khaddour K. Tumor Necrosis Factor Inhibitors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Jan 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482425/
An Overview of Cytokines and Cytokine Antagonists as Therapeutic Agents - Donnelly - 2009 - Annals of the New York Academy of Sciences - Wiley Online Library [Internet]. [cited 2024 Jan 26]. Available from: https://nyaspubs.onlinelibrary.wiley.com/doi/https://doi.org/10.1111/j.1749-6632.2009.05382.x
Anakinra: Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/anakinra-drug-information?search=anakinra&source=panel_search_result&selectedTitle=1~128&usage_type=panel&kp_tab=drug_general&display_rank=1#F135437
Etanercept (including biosimilars available in Canada): Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/etanercept-including-biosimilars-available-in-canada-drug-information?search=etanercept&source=panel_search_result&selectedTitle=1~149&usage_type=panel&kp_tab=drug_general&display_rank=1#references
Infliximab (including biosimilars): Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/infliximab-including-biosimilars-drug-information?search=infliximab&source=panel_search_result&selectedTitle=1~149&usage_type=panel&kp_tab=drug_general&display_rank=1
Adalimumab (including biosimilars): Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/adalimumab-including-biosimilars-drug-information?search=adalimumab&source=panel_search_result&selectedTitle=1~149&usage_type=panel&kp_tab=drug_general&display_rank=1
Certolizumab pegol: Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/certolizumab-pegol-drug-information?search=certolizumab%20pegol&source=panel_search_result&selectedTitle=1~68&usage_type=panel&kp_tab=drug_general&display_rank=1
Golimumab: Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/golimumab-drug-information?search=golimumab&source=panel_search_result&selectedTitle=1~67&usage_type=panel&kp_tab=drug_general&display_rank=1#F7764845
Sarilumab: Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/sarilumab-drug-information?search=sarilumab&source=panel_search_result&selectedTitle=1~23&usage_type=panel&kp_tab=drug_general&display_rank=1
Tocilizumab (including biosimilars): Drug information - UpToDate [Internet]. [cited 2024 Jan 26]. Available from: https://www.uptodate.com/contents/tocilizumab-including-biosimilars-drug-information?source=auto_suggest&selectedTitle=1~1---1~4---tocili&search=tocilizumab
Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J. 2012;21(10):2079–84. https://doi.org/10.1007/s00586-012-2183-5.
Bessette L, Haraoui B, Chow A, Fortin I, Dixit S, Khraishi M, et al. Effectiveness and safety of certolizumab pegol in rheumatoid arthritis patients in Canadian practice: 2-year results from the observational FαsT-CAN study. Ther Adv Musculoskelet Dis. 2019;5(11):1759720X19831151. https://doi.org/10.1177/1759720X19831151.
Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67(6):1424–37. https://doi.org/10.1002/art.39093.
Aitken D, Laslett LL, Pan F, Haugen IK, Otahal P, Bellamy N, et al. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis—the HUMOR trial. Osteoarthritis Cartilage. 2018;26(7):880–7. https://doi.org/10.1016/j.joca.2018.02.899.
Cohen SB, Proudman S, Kivitz AJ, Burch FX, Donohue JP, Burstein D, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. 2011;13(4):R125. https://doi.org/10.1186/ar3430.
Hung AL, Lim M, Doshi TL. Targeting cytokines for treatment of neuropathic pain. Scand J Pain. 2017;17:287–93. https://doi.org/10.1016/j.sjpain.2017.08.002.
Kuffler DP. Mechanisms for Reducing Neuropathic Pain. Mol Neurobiol. 2020;57(1):67–87. https://doi.org/10.1007/s12035-019-01757-9.
Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–45. https://doi.org/10.1038/s41584-020-0419-z.
Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62(2):542–52. https://doi.org/10.1002/art.27221.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors listed have made a direct and intellectual contribution to the work and have been approved for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Dominique M. Perilloux, Alison M. Hawkins, Grace C. Wester, Amanda R. Ragaland, Sage V. Hebert, Julian Kim, Michael Heisler, Rucha A. Kelkar, Azem A. Chami, Sahar Shekoohi, and Adam M. Kaye have nothing to disclose. Alan Kaye is an Editorial Board member of Pain and Therapy. Alan Kaye was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previous studies and contains no new studies with human participants or animals performed by any authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kaye, A.D., Perilloux, D.M., Hawkins, A.M. et al. Tumor Necrosis Factor and Interleukin Modulators for Pathologic Pain States: A Narrative Review. Pain Ther 13, 481–493 (2024). https://doi.org/10.1007/s40122-024-00603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00603-8