Abstract
Purpose of review
Osteoarthritis (OA) is the most common form of arthritis, and pain is the primary symptom of the disease, yet analgesic options for treating OA pain remain limited. In this review, we aimed to give an update on the current clinical and preclinical studies targeting two pathways that are being investigated for treating OA pain: the nerve growth factor (NGF) pathway and the transient receptor potential vanilloid-1 (TRPV1) pathway.
Recent findings
Antibodies against NGF, small molecule inhibitors of TrkA, TRPV1 agonists, and TRPV1 antagonists are all in different stages of clinical and pre-clinical testing for the treatment of OA pain. NGF antibodies have shown efficacy in the primary endpoints tested compared with placebo; however, rapidly progressive OA has been consistently observed in a subset of patients and the cause remains unclear. TRPV1 agonists have also demonstrated reduced pain with no serious adverse events—the most common adverse events include a burning or warming sensation upon administration.
Summary
Targeting the NGF and TRPV1 pathways appears effective for reducing OA pain, but further work is needed to better understand which patients may benefit most from these treatments. The anti-NGF antibody tanezumab and the TRPV1 agonist CNTX-4975 have both received fast-track designation from the FDA for the treatment of OA pain.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. https://doi.org/10.1016/S0140-6736(18)32279-7.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
Osteoarthritis: a serious disease. https://www.oarsi.org/research/oa-serious-disease: Pre Competitive Consortium for Osteoarthritis Osteoarthritis Research Society International 2016.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis & Rheumatology.n/a(n/a). doi:https://doi.org/10.1002/art.41142.
Osani MC, Vaysbrot EE, Zhou M, McAlindon TE, Bannuru RR. Duration of symptom relief and early trajectory of adverse events for oral NSAIDs in knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care & Research. https://doi.org/10.1002/acr.23884.
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1145–53. https://doi.org/10.1016/j.joca.2013.03.018.
Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(4):682–8. https://doi.org/10.1136/annrheumdis-2013-204191.
Carlesso LC, Segal NA, Frey-Law L, Zhang Y, Na L, Nevitt M, et al. Pain susceptibility phenotypes in those free of knee pain with or at risk of knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2019;71(4):542–9. https://doi.org/10.1002/art.40752.
Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9(11):654–64. https://doi.org/10.1038/nrrheum.2013.138.
Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64(9):2907–16. https://doi.org/10.1002/art.34466.
Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88(1):69–78. https://doi.org/10.1016/s0304-3959(00)00310-9.
Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156(1):55–61. https://doi.org/10.1016/j.pain.0000000000000022.
Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21(7):695–703. https://doi.org/10.1080/14728222.2017.1328057.
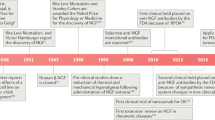
Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci. 2017;40:307-25. doi:https://doi.org/10.1146/annurev-neuro-072116-031121.This review provides background on the NGF signaling pathway.
Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115(1):189–204. https://doi.org/10.1097/ALN.0b013e31821b1ac5.
Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66(11):3018–27. https://doi.org/10.1002/art.38778.
Manni L, Lundeberg T, Fiorito S, Bonini S, Vigneti E, Aloe L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol. 2003;21(5):617–24.
Iannone F, De Bari C, Dell'Accio F, Covelli M, Patella V, Lo Bianco G, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford). 2002;41(12):1413–8. https://doi.org/10.1093/rheumatology/41.12.1413.
Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852–61. https://doi.org/10.1093/rheumatology/keq188.
Aso K, Shahtaheri SM, Hill R, Wilson D, McWilliams DF, Walsh DA. Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol. 2019;71(6):916–24. https://doi.org/10.1002/art.40820.
Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–31. https://doi.org/10.1056/NEJMoa0901510.
Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthr Cartil. 2015;23(Suppl 1):S18–21. https://doi.org/10.1016/j.joca.2014.10.005.
Miller RE, Block JA, Malfait AM. What is new in pain modification in osteoarthritis? Rheumatology (Oxford). 2018;57(suppl_4):iv99-iv107. doi:https://doi.org/10.1093/rheumatology/kex522.
Tive L, Bello AE, Radin D, Schnitzer TJ, Nguyen H, Brown MT, et al. Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase III clinical trials in patients with osteoarthritis pain of the knee or hip. J Pain Res. 2019;12:975–95. https://doi.org/10.2147/JPR.S191297.
Chen J, Li J, Li R, Wang H, Yang J, Xu J et al. Efficacy and safety of tanezumab on osteoarthritis knee and hip pains: a meta-analysis of randomized controlled trials. Pain Medicine. 2016:pnw262. doi:https://doi.org/10.1093/pm/pnw262.
Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthr Cartil. 2015;23:S8–S17. https://doi.org/10.1016/j.joca.2014.10.003.
Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, et al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol. 2016;68(2):382–91. https://doi.org/10.1002/art.39492.
Schnitzer TJ, Ekman EF, Spierings ELH, Greenberg HS, Smith MD, Brown MT, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis. 2015;74(6):1202–11. https://doi.org/10.1136/annrheumdis-2013-204905.
Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. 2019;322(1):37–48. doi:https://doi.org/10.1001/jama.2019.8044. This is the most recent clinical trial demonstrating efficacy of tanezumab compared with placebo over 16 weeks.
Hochberg M, Carrino J, Schnitzer T, Guermazi A, Vignon E, Walsh D et al. Subcutaneous tanezumab vs NSAID for the treatment of osteoarthritis: efficacy and general safety results from a randomized, double-blind, active-controlled, 80-week, phase-3 study [abstract]. Arthritis Rheum. 2019;71(suppl 10):1302. https://acrabstracts.org/abstract/subcutaneous-tanezumab-vs-nsaid-for-the-treatment-of-osteoarthritis-efficacy-and-general-safety-results-from-a-randomized-double-blind-active-controlled-80-week-phase-3-study/.
Hochberg M, Carrino J, Schnitzer T, Guermazi A, Walsh D, White A et al. Subcutaneous tanezumab versus NSAID for the treatment of osteoarthritis: joint safety events in a randomized, double-blind, active-controlled, 80-week, phase-3 study [abstract]. Arthritis Rheum. 2019;71(suppl 10):2756. https://acrabstracts.org/abstract/subcutaneous-tanezumab-vs-nsaid-for-the-treatment-of-osteoarthritis-efficacy-and-general-safety-results-from-a-randomized-double-blind-active-controlled-80-week-phase-3-study/.
Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on-study safety of potential shoulder adverse events in anti-NGF studies (part 3). Osteoarthr Cartil. 2015;23(Suppl 1):S59–68. https://doi.org/10.1016/j.joca.2014.09.018.
Dakin P, DiMartino SJ, Gao H, Maloney J, Kivitz AJ, Schnitzer TJ et al. The efficacy, tolerability, and joint safety of fasinumab in osteoarthritis pain: a phase IIb/III double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheumatol. 2019;71(11):1824–34. https://doi.org/10.1002/art.41012. This is the most recent clinical trial demonstrating efficacy of fasinumab compared with placebo over 16 weeks.
Mayorga AJ, Wang S, Kelly KM, Thipphawong J. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomised, placebo- and active-controlled trial. Int J Clin Pract. 2016;70(6):493–505. https://doi.org/10.1111/ijcp.12807.
Krupka E, Jiang GL, Jan C. Efficacy and safety of intra-articular injection of tropomyosin receptor kinase A inhibitor in painful knee osteoarthritis: a randomized, double-blind and placebo-controlled study. Osteoarthr Cartil. 2019;27(11):1599–607. https://doi.org/10.1016/j.joca.2019.05.028.
Watt FE, Blauwet MB, Fakhoury A, Jacobs H, Smulders R, Lane NE. Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: results from a randomized controlled phase 2a trial. Osteoarthr Cartil. 2019;27(11):1590–8. https://doi.org/10.1016/j.joca.2019.05.029.
Walsh DA, Neogi T. A tale of two TrkA inhibitor trials: same target, divergent results. Osteoarthritis and cartilage. 2019;27(11):1575-7. https://doi.org/10.1016/j.joca.2019.07.013. This editorial provides discussion on mixed results in two TrkA inhibitor clinical trials.
Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthr Cartil. 2015;23(6):925–32. https://doi.org/10.1016/j.joca.2015.02.002.
Bryden LA, Nicholson JR, Doods H, Pekcec A. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: an objective measure of pain-related behavior and analgesic efficacy. Osteoarthr Cartil. 2015;23(9):1605–12. https://doi.org/10.1016/j.joca.2015.05.001.
Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Inoue G, Sakuma Y, et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskeletal Disorders. 2017;18(1). https://doi.org/10.1186/s12891-017-1792-x.
Sakurai Y, Fujita M, Kawasaki S, Sanaki T, Yoshioka T, Higashino K, et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. PAIN. 2019;160(4):895–907. https://doi.org/10.1097/j.pain.0000000000001466.
McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149(2):386–92. https://doi.org/10.1016/j.pain.2010.03.002.
Xu L, Nwosu LN, Burston JJ, Millns PJ, Sagar DR, Mapp PI, et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthr Cartil. 2016;24(9):1587–95. https://doi.org/10.1016/j.joca.2016.05.015.
LaBranche TP, Bendele AM, Omura BC, Gropp KE, Hurst SI, Bagi CM et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Annals of the rheumatic diseases. 2016;76(1):295-302. https://doi.org/10.1136/annrheumdis-2015-208913. A preclinical study in the rat MMT model saw accelerated cartilage damage with anti-NGF therapy.
von Loga IS, El-Turabi A, Jostins L, Miotla-Zarebska J, Mackay-Alderson J, Zeltins A, et al. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann Rheum Dis. 2019;78(5):672–5. https://doi.org/10.1136/annrheumdis-2018-214489.
Nwosu LN, Mapp PI, Chapman V, Walsh DA. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Annals of the rheumatic diseases. 2015;75(6):1246-54. https://doi.org/10.1136/annrheumdis-2014-207203. A preclinical study demonstrated analgesic efficacy using a TrkA inhibitor in two models of OA.
Sousa-Valente J, Calvo L, Vacca V, Simeoli R, Arévalo JC, Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr Cartil. 2018;26(1):84–94. https://doi.org/10.1016/j.joca.2017.08.006.
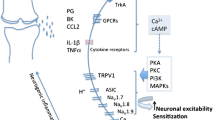
Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155(8):1145–62. https://doi.org/10.1038/bjp.2008.351. This review provides background on the TRPV1 signaling pathway.
Schumacher MA. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010;10(3):185–200. https://doi.org/10.1111/j.1533-2500.2010.00358.x.
Persson MSM, Stocks J, Walsh DA, Doherty M, Zhang W. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis and cartilage. 2018;26(12):1575-82. https://doi.org/10.1016/j.joca.2018.08.008. A systematic review describes the efficacy of topical capsaicin clinical trials.
Billard M TJ, Fleming M, Warneke T, Qiu Y, Ly N, Aronstein W, Moore W. A phase 2 double-blind clinical trial to examine the comparative E. Arthritis Rheum. 2019;71(suppl 10).
Stevens RM, Ervin J, Nezzer J, Nieves Y, Guedes K, Burges R et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis & Rheumatology. 2019;71(9):1524–33. https://doi.org/10.1002/art.40894. This is the most recent clinical trial demonstrating efficacy of CNTX-4975 compared with placebo.
Schnitzer TJ, Pelletier JP, Haselwood DM, Ellison WT, Ervin JE, Gordon RD, et al. Civamide cream 0.075% in patients with osteoarthritis of the knee: a 12-week randomized controlled clinical trial with a longterm extension. J Rheumatol. 2012;39(3):610–20. https://doi.org/10.3899/jrheum.110192.
Appendino G, Szallasi A. Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997;60(10):681–96. https://doi.org/10.1016/s0024-3205(96)00567-x.
Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, et al. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J Clin Invest. 2018;128(4):1657–70. https://doi.org/10.1172/JCI94331.
Arsenault P, Chiche D, Brown W, Miller J, Treister R, Leff R, et al. NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018;3(6):e696. https://doi.org/10.1097/PR9.0000000000000696.
Mayorga AJ, Flores CM, Trudeau JJ, Moyer JA, Shalayda K, Dale M, et al. A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand J Pain. 2017;17:134–43. https://doi.org/10.1016/j.sjpain.2017.07.021.
Manitpisitkul P, Flores CM, Moyer JA, Romano G, Shalayda K, Tatikola K, et al. A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand J Pain. 2018;18(2):151–64. https://doi.org/10.1515/sjpain-2017-0184.
Kalff K-M, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, et al. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010;641(2–3):108–13. https://doi.org/10.1016/j.ejphar.2010.05.022.
Kim Y, Kim E-h, Lee KS, Lee K, Park SH, Na SH et al. The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats. The Korean Journal of Physiology & Pharmacology. 2016;20(1):129. https://doi.org/10.4196/kjpp.2016.20.1.129.
Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain. 2018;159(10):2105–14. https://doi.org/10.1097/j.pain.0000000000001314.
Abaei M, Sagar DR, Stockley EG, Spicer CH, Prior M, Chapman V, et al. Neural correlates of hyperalgesia in the monosodium iodoacetate model of osteoarthritis pain. Mol Pain. 2016;12:174480691664244. https://doi.org/10.1177/1744806916642445.
Haywood AR, Hathway GJ, Chapman V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci Rep. 2018;8(1):7122. https://doi.org/10.1038/s41598-018-25581-8.
Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314(1):410–21. https://doi.org/10.1124/jpet.105.083915.
Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, et al. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142(1–2):27–35. https://doi.org/10.1016/j.pain.2008.11.004.
Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26(37):9385–93. https://doi.org/10.1523/JNEUROSCI.1246-06.2006.
Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, et al. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydr onaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150(2):319–26. https://doi.org/10.1016/j.pain.2010.05.015.
Chu KL, Chandran P, Joshi SK, Jarvis MF, Kym PR, McGaraughty S. TRPV1-related modulation of spinal neuronal activity and behavior in a rat model of osteoarthritic pain. Brain Res. 2011;1369:158–66. https://doi.org/10.1016/j.brainres.2010.10.101.
Voight EA, Gomtsyan AR, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK, et al. Discovery of (R)-1-(7-chloro-2,2-bis (fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl) ur ea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy. J Med Chem. 2014;57(17):7412–24. https://doi.org/10.1021/jm500916t.
Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153(4):924–33. https://doi.org/10.1016/j.pain.2012.01.022.
Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015;74(1):252–9. https://doi.org/10.1136/annrheumdis-2013-203413.
Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 2008;58(1):145–53. https://doi.org/10.1002/art.23156.
Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, et al. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr Cartil. 2010;18(11):1536–43. https://doi.org/10.1016/j.joca.2010.09.005.
Fowler CJ, Naidu PS, Lichtman A, Onnis V. The case for the development of novel analgesic agents targeting both fatty acid amide hydrolase and either cyclooxygenase or TRPV1. Br J Pharmacol. 2009;156(3):412–9. https://doi.org/10.1111/j.1476-5381.2008.00029.x.
Malek N, Mrugala M, Makuch W, Kolosowska N, Przewlocka B, Binkowski M, et al. A multi-target approach for pain treatment: dual inhibition of fatty acid amide hydrolase and TRPV1 in a rat model of osteoarthritis. Pain. 2015;156(5):890–903. https://doi.org/10.1097/j.pain.0000000000000132.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Obeidat, A.M., Donner, A. & Miller, R.E. An Update on Targets for Treating Osteoarthritis Pain: NGF and TRPV1. Curr Treat Options in Rheum 6, 129–145 (2020). https://doi.org/10.1007/s40674-020-00146-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-020-00146-x