Abstract
Introduction
This study aims to evaluate the efficacy of esketamine on postoperative recovery quality after laparoscopic bariatric surgery.
Methods
Patients (n = 74) scheduled for laparoscopic bariatric surgery were randomly divided into two groups: the esketamine group (group E: 0.5 mg/kg/h infusion, i.e., 0.2 mL/kg/h) or the control group (group C: 0.2 mL/kg/h normal saline infusion). The infusions were stopped 20 min before the end of the procedure. The primary outcome was the Quality of Recovery-40 (QoR-40) score on postoperative day 1 (POD 1). The secondary outcomes included QoR-40 scores on PODs 2 and 7, Numeric Rating Scale (NRS) on PODs 1, 2, and 7, time to extubation, additional postoperative analgesic use, length of hospital stay, and time to first exhaust. Additonally, the safety indices were also recorded, including hemodynamic profile, perioperative anesthesia index (Ai), utilization of vasoactive drugs or urapidil, and side effects.
Results
All in all, 70 of the 74 patients completed the study, 35 in each group. The difference of QoR-40 scores on POD 1 was both statistically and clinically significant [difference 7.21, 95% confidence interval (CI) 5.17, 9.25, p < 0.001]. The difference of QoR-40 on POD 2 was statistically significant but clinically insignificant (difference 4.81, 95% CI 2.69, 6.92, p < 0.001). The difference of NRS scores on POD 1 was statistically significant (difference −1.23, 95% CI −2.36, −0.10, p = 0.033). Compared with group C, group E had a lower utilization rate of phenylephrine and higher Ai values (p < 0.05). There was no statistical difference between the two groups on other measures.
Conclusion
Continuous ketamine infusion seems to be safe and well tolerated in laparoscopic bariatric surgery. It improved the quality of postoperative recovery and reduced pain on POD 1. In spite of the increased Ai value during the surgery, it also provided better hemodynamics with less usage of phenylephrine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While laparoscopic bariatric surgery is an effective treatment for obesity, postoperative pain is still a common occurrence, despite advances in minimally invasive techniques. Opioid-related adverse reactions limit its early postoperative use. |
Esketamine, as an isomer of ketamine, has good sedation and analgesia, while preserving respiration and airway reflex. It is reported to have higher potency and a lower risk of adverse reactions than racemic ketamine. |
We hypothesized that esketamine could reduce the postoperative pain and improve postoperative recovery quality. |
What was learned from the study? |
Esketamine infusion administered at a dose of 0.5 mg/kg/h was safe and effective at improving the quality of postoperative recovery and reducing pain on postoperative day 1. It also provided better hemodynamics with less usage of phenylephrine. |
Introduction
Bariatric surgery is one of the most effective and cost-effective treatment options for obese people [1]. Although laparoscopic surgery is less painful than open surgery, opioids are still indispensable for perioperative analgesia [2]. However, opioids also induce many side effects, such as itching, urinary retention, nausea and vomiting, respiratory depression, and even death [3]. A previous study reported that 70% of patients with obesity suffer from obstructive sleep apnea (OSA) [4]. The characteristic significantly reduces their ability to tolerate hypoxia, thus limiting the use of opioids. Therefore there is growing support for the use of multimodal analgesia in this population to optimize perioperative analgesia.
Ketamine, a receptor antagonist of N-methyl-d-aspartic acid (NMDA), has good sedation and analgesia while preserving respiration and airway reflex [5,6,7]. Recent literature has proved that ketamine can decrease postoperative pain scores and opioid consumption following bariatric surgery, making it an alternative option for the opioid reduction strategy [8]. Notably, the drug has often been questioned regarding its psychiatric adverse reactions [9].
As an isomer of ketamine, esketamine is reported to have higher potency and lower risk of adverse reactions than racemic ketamine [10]. The safety and effectiveness of esketamine ensure its broad prospects for clinical practice. Previous findings reported that esketamine improved postoperative recovery quality in modified radical mastectomy and video-assisted thoracic surgery [11,12,13]. To our knowledge, no research has been performed on the efficacy of esketamine on recovery after laparoscopic bariatric surgery (LBS). Here, we conducted a randomized controlled trial and used the Quality of Recovery-40 (QoR-40) questionnaire to evaluate whether esketamine infusion could enhance postoperative recovery in patients undergoing LBS.
Methods
Study
The Ethics Committee of The Affiliated Hospital of Xuzhou Medical University has authorized the prospective, randomized controlled trial (XYFY2022-KL037-02). The trial was registered on the Chinese Clinical Trial Registry prior to initiation (ChiCTR2200066138). The study was in accordance with the Declaration of Helsinki and its later amendments. All patients provided their written informed consent. The trial report complies with the Consolidated Standards of Reporting Trials (CONSORT) checklist (Supplementary Material, Table S1).
Participants
Patients were between 18 and 65 years old, with Body Mass Index (BMI) over 30 kg/m2, American Society of Anesthesiologists (ASA) classification II–III, and were scheduled for LBS. The study’s exclusion criteria included: (a) allergy to any medication used in the study, (b) known hyperthyroidism or pheochromocytoma, grade III hypertension (very high risk), severe arrhythmias, severe cardio-cerebrovascular disease, or hemodynamic instability, (c) severe hepatorenal insufficiency, (d) recent use of any tricyclic antidepressants, sedatives, opioids, monoamine oxidase inhibitors, or sleep-inducing medications within the past month, (e) anemia with hemoglobin levels less than 9.0 mg/dL, and (f) refusal to provide informed consent or inability to cooperate with the researcher.
Randomization, Blinding, and Concealment
Each individual was randomly assigned on the basis of a random table from http://www.randomization.com in a 1:1 ratio to either the esketamine group (group E) or the control group (group C). The patient’s allocation was placed in an opaque and sealed envelope. Upon admission to the surgical center, the envelope was opened by a separate anesthetist who had no involvement in patient care or data collection. This anesthetist was responsible for preparing 20 mL syringes of 0.9% normal saline with or without 50 mg esketamine, which run at a rate of 0.2 mL/kg/h during surgery. Besides, emergency envelopes containing the case medication number and drug name were prepared to enable the unblinding and resuscitation if necessary. Other staff members could not identify the group of preparation in light of its appearance. Surgical team members, anesthetists, data collectors, and statisticians were blinded to the group allocation. The trial’s surgical procedures were conducted exclusively by a single surgical team.
Intraoperative and Postoperative Management
All patients were required to fast for 8 h before surgery. After they were admitted to the operating room, electrocardiography, heart rate, invasive blood pressure, blood oxygen saturation (SpO2), anesthesia index (Ai), and train-of-four stimulation (TOF) were routinely monitored. Ai with a range from 0 to 100, was monitored by Pearlcare Anesthesia Depth Monitor (Conview YY-105). A low value indicated deeper anesthesia. After preoxygenation for 5 min, anesthesia was induced with 0.05 mg/kg midazolam, 0.5 µg/kg sufentanil, 0.3 mg/kg etomidate, rocuronium 0.8 mg/kg, and dezocine 5 mg. All drug dosage calculations were based on lean body weight (LBW). The patient was intubated using an endotracheal tube upon a TOF of 0 and an Ai of 60. After intubation, the ventilation mode was changed to pressure-control-volume-guaranteed (PCV-VG) mode: oxygen concentration 60%, oxygen flow 2 L/min, positive end-expiratory pressure 5 cmH2O, partial pressure of end-tidal carbon dioxide (PetCO2) 35–40 mmHg. All patients received transverse abdominal plane (TAP) blocks with 20 mL of 0.25% ropivacaine on each side under ultrasound guidance.
General anesthesia was maintained with 1% sevoflurane, propofol at 4–6 mg/kg/h, and remifentanil at 0.1–0.3 μg/kg/min. Infusions of the study medications were followed by endotracheal intubation. Group E received 0.5 mg/kg/h esketamine (i.e., 0.2 mL/kg/h) infusion. Group C received 0.2 mL/kg/h normal saline. The infusions were stopped 20 min before the end of the procedure. During surgery, the infusion rate of propofol or remifentanil was adjusted by an experienced anesthesiologist to maintain mean arterial pressure (MAP) within ± 20% of baseline values and an Ai value within 40–60. In case of mean blood pressure (MBP) below 30% of baseline values, injections of 3 mg ephedrine [heart rate (HR) < 50 beats/min] or 40 μg phenylephrine (HR ≥ 50 beats/min) were given; when MBP was above 30% of baseline values, 5 mg urapidil was injected. In case of bradycardia (HR < 50 beats/min), 0.5 mg atropine was administered. These measures would be repeated as necessary. Rocuronium or cisatracurium was titrated on the basis of TOF, surgeon demand, or post-tetanic count over 15. Pneumoperitoneum inflation pressure ranged from 12 to 15 mmHg.
For both groups, all patients received an intravenous infusion of crystalloid and colloid solution in a 2:1 ratio. All subjects received systematic prophylaxis for nausea and vomiting, including dexamethasone 5 mg and phencyclidine hydrochloride 10 mg before the operation and palonosetron 0.25 mg at the end of the operation. Ketorolac 60 mg was given intravenously 20 min before the end of surgery. Upon skin closure, inhaled drugs ceased, and oxygen flow was increased to 8 L/min to hasten sevoflurane washout. Once closure has been completed, propofol and remifentanil were discontinued, and 0.5 mg flumazenil was injected intravenously. The residual neuromuscular block was antagonized by 0.04 mg/kg neostigmine and 0.02 mg/kg atropine. All patients were extubated in the operating room when T4/T1 was > 90% and tidal volume was > 5 mL/kg and then transported to the postanesthesia care unit (PACU).
In PACU, patients received standard postoperative monitoring and 2–3 L/min oxygen by nasal cannula. If the NRS score exceeded 4 points, 5 mg of dezocine would be injected intravenously. If SpO2 ≤ 90%, patients were alerted by waking them up and asking them to breathe deeply. Supplemental oxygen of the mask was initiated at 2 L/min if the respiratory rate was less than 8. The patients were transferred to the ward upon Modified Aldrete Score (MAS) above 9 points. On the surgical ward, ketorolac 30 mg was routinely administered intravenously every 8 h and use of dezocine depended on the patient’s needs. Patients were encouraged to early ambulation and to take a deep breath on the POD 1.
Outcome measures
The primary outcome was the QoR-40 score assessed on postoperative day 1 (POD 1). The questionnaire evaluates five components of patient recovery: physical comfort (12 questions), physical independence (5 questions), emotional state (9 questions), psychological support (7 questions), and pain (10 questions). The QoR-40 questionnaire is scored on a scale of 40–200. Each patient was provided with a detailed explanation of all questions in the QoR-40 questionnaire on the day prior to surgery.
The secondary outcomes included time to extubation, additional postoperative analgesic use, QoR-40 on PODs 2 and 7, Numeric Rating Scale (NRS) on PODs 1, 2 and 7, length of hospital stay, and time to first exhaust (the interval between the end of surgery and the first flatulence). Patients who were discharged prior to POD 7 were contacted by mail or telephone by a team member to complete the QoR-40 and NRS questionnaires.
The safety indices were the utilization of vasoactive drugs or urapidil and the incidence of side effects (PONV, urinary retention, itching, or adverse central nervous system events). In addition, MAP, HR, and Ai were recorded at baseline (T0), 5 min (T1), 10 min (T2), 15 min (T3) and 30 min (T4) after intubation, at extubation (T5), and 5 min after extubation (T6).
Sample size
The sample size was calculated based on the results of the pilot study (unpublished data, n = 20) using PASS version 15.0. The mean QoR-40 scores in the ketamine and control groups were 175.0 and 168.7, the standard deviations (SDs) were 7.46 and 6.99, respectively. Assuming an α of 0.05, β of 0.1, and a dropout rate of 20%, with a two-tailed analysis, each group required 37 patients in this trial. Eventually, 74 cases were needed to be recruited for this study.
Statistical Analysis
We performed the data analysis with Statistical Package for Social Sciences (SPSS) software version 26.0 and GraphPad Prism version 8.0. A two-sided p < 0.05 was considered statistically significant. The statistical analysis was performed per protocol. For continuous data, the Shapiro–Wilk test, combined with histograms, was used to determine the normality of data. Normally distributed data were reported as mean ± SD and evaluated using a two-sided t-test for equal variances. Non-normally distributed interval and ordinal data were presented as median (interquartile range) and evaluated using the Mann–Whiney U test. Categorical variables were summarized as absolute numbers (%) and assessed using a chi-squared test (χ2) and Fisher’s exact test.
Repeated normally distributed variables with a confounding variable (HR, MAP, Ai) were assessed by analysis of covariance (ANCOVA). The covariates were the baseline measurement. The sphericity was assessed with Mauchly’s test, and if the assumption of sphericity was violated, the Greenhouse–Geisser correction would be used for correcting the degrees of freedom. For repeated abnormally distributed variables (NRS, QoR-40), a generalized estimating equation (GEE) was used for comparison.
We conducted multiple linear regression analyses to assess the association between QoR-40 scores on POD 1 and various factors. We checked for multicollinearity among the independent variables using variance inflation factor (VIF), and considered a VIF > 10 to indicate statistically significant multicollinearity. Our independent variables included age, BMI, patient sex, ASA status, surgery type, surgery time, intraoperative sevoflurane and remifentanil doses, preoperative QoR-40 score, and group allocation. We performed univariate linear regression to identify variables with p < 0.1, which were then included in subsequent multivariate linear regression analyses.
Post hoc exploratory subgroup analyses of the primary endpoint were also performed according to age, sex, BMI, surgical type, and ASA grade. The subgroup results were presented in the form of mean ± SD and interaction p values.
Results
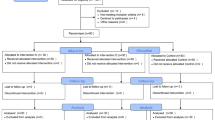
Initially, a total of 92 patients who underwent LBS at our hospital between 15 July 2022 and 30 November 2022 were screened. Of them, 10 patients failed the inclusion criteria, 8 patients declined to participate, and 74 patients were enrolled. Each group had two patients lost to follow-up. The missing data accounted for 5.4%. We carried out per-protocol analysis and applied the complete case analysis method for handling missing data. In total, 70 (94.6%) patients were statistically analyzed, with 35 patients per group (Fig. 1). There were no significant differences in baseline characteristics or intraoperative data between two groups (p > 0.05), except for a lower incidence of the utilization of phenylephrine in the esketamine group (20% for the group E versus 46% for group C, p < 0.05) (Table 1).
Recovery quality (QoR-40) score changes
The changes in the QoR-40 score over time are shown in Fig. 2a. We carried out a generalized estimated equation (GEE) to compare the two groups in overall QoR-40 on PODs 1, 2, and 7 after correcting the effect of baseline QoR-40 (Table 2). There was an interaction between time and group (p < 0.001). The QoR-40 on PODs 1 and 2 were significantly higher in group E than in group C (p < 0.001), and there was no difference in QoR-40 on POD 7 (p = 0.745). The estimated differences of QoR-40 on PODs 1, 2, and 7 between group E and C were 7.21 (95% CI 5.17, 9.25), 4.81 (95% CI 2.69, 6.92), and −0.28 (95% CI −1.95, 1.39), respectively. More details are shown in Table 2.
In terms of individual domains, Group E showed a significantly higher improvement with regard to emotional and pain dimensions on POD 1 than Group C (p < 0.05). There was no significant difference in all other dimensional QoR-40 scores (p > 0.05) (Supplementary Material, Table S2).
Postoperative NRS Score Changes
The changes in NRS scores over time are shown in Fig. 2b. We carried out a generalized estimated equation (GEE) to compare the two groups in NRS score on PODs 1, 2, and 7 (Supplementary Material, Table S3). The estimated difference of NRS on POD 1 was −1.23 (95% CI −2.36, −0.10, p = 0.033 ) between groups E and C. There was no significant difference in NRS scores on PODs 2 and 7 (p = 0.067 and p = 0.873, respectively).
Perioperative Hemodynamic and Ai Changes
The change in MAP over time is shown in Fig. 3a. According to Mauchly’s test, the sphericity assumption was violated (W = 0.072, p < 0.001). That means the degrees of freedom would be corrected using Greenhouse–Geisser estimates of sphericity (ε). The main effects of the group and the interaction effect between time and group were not significant (F = 1.510, p = 0.223, and F = 1.360, p = 0.257, respectively). Overall, there was no statistical difference in MAP between the two groups.
a Perioperative mean arterial pressure changes. b Perioperative heart rate changes. c Perioperative Ai changes. Values are presented as mean ± standard deviation and data were analyzed using ANCOVA. Group E esketamine group, Group C control group, POD postoperative day, T0 at baseline before anesthetic induction, T1 5 min after the induction, T2 10 min after the induction, T3 15 min after the induction, T4 30 min after the induction, T5 at tracheal extubation, T6 5 min after tracheal extubation. *p < 0.05
The change in HR over time is shown in Fig. 3b. According to Mauchly’s test, the sphericity assumption was violated (W = 0.143, p < 0.001). That means the degrees of freedom would be corrected using Greenhouse–Geisser estimates of sphericity (ε). The main effects of the group and the interaction effect between time and group were not significant (F = 1.619, p = 0.208, and F = 0.176, p = 0.892, respectively ). Overall, there was no statistical difference in HR between the two groups.
The change in Ai over time is shown in Fig. 3c. According to Mauchly’s test, the sphericity assumption was violated (W = 0.398, p < 0.001). That means the degrees of freedom would be corrected using Greenhouse–Geisser estimates of sphericity (ε). The main effect of the group was significant (F = 22.153, p < 0.001). There was no interaction between time and group (F = 2.089, p = 0.074). Compared with values in group C, Ai was higher at T2, T3, T4, and T5 in group E (p < 0.05).
Recovery Characteristics and Postoperative Complications
Table 3 summarizes the patient’s condition after the operation. While time to extubation was less in group E than in group C, the difference was insignificant (p = 0.420). In PACU, there were four patients with CNS adverse events in group E, and five in group C (11% versus 14%, p > 0.05).In group E, three patients experienced vivid dreams and one experienced agitation. In group C, one patient experienced vivid dreams, one felt confused and disoriented, and three experienced agitation. No significant differences were identified with regard to supplemental oxygen in PACU, dezocine dose, PONV, length of hospital stay, and urinary retention between the two groups (p > 0.05). There were no major adverse respiratory events and opioid-related itching in the two groups after surgery.
Multiple Linear Regression Analysis for QoR-40 Score on POD 1
The univariate linear regression analysis demonstrated preoperative QoR-40, grouping (p < 0.1). Subsequently, the multivariate linear regression analysis revealed no multicollinearity between preoperative QoR-40 and grouping (VIF = 1.001), and both were positively associated with the quality of recovery on POD 1 (p < 0.001). More details are shown in Supplementary Material, Table S4.
Subgroups Analysis of QoR-40 on POD 1
Post hoc exploratory subgroup analyses were performed to detect any subgroups in which effect could be observed, but we did not find any interaction between grouping factors and subgroup classifications (Supplementary Material, Table S5).
Discussion
The clinical advantages and safety of esketamine have been reported in many studies [11, 14,15,16,17,18,19,20,21], but none of these assessed postoperative recoveries after bariatric surgery. Our trial took the QoR-40 questionnaire as a measurement of postoperative recovery. A recent study reported that the minimum clinically important difference (MCID) of QoR-40 was 6.3 [22]. In other words, the change resulting from perioperative interventions can be interpreted as a meaningful variation in health status. Our study demonstrated a statistically and clinically important difference of QoR-40 on POD 1 in favor of esketamine (difference, 7.21). Furthermore, in five dimensions of QoR-40, esketamine group had significantly better scores in emotional state and pain on POD 1. The difference of QoR-40 on POD 2 was statistically significant, but clinically insignificant (difference, 4.81). This also explains why there was no difference in the five-dimensional scores on POD 2.
Min Zhu et al.’s [11] trial found that 4 μg/kg/h esketamine administration significantly reduced postoperative pain on POD 1 in patients undergoing modified radical mastectomy. Miziara et al. [21] reported that esketamine infusion alleviated postoperative pain undergoing laparoscopic cholecystectomy. Our results are consistent with these results. We observed that esketamine reduced the pain score on the first day after surgery, in line with the pain dimension score of QoR-40. Nevertheless, the findings also indicated that the significant reduction in pain scores did not persist after POD 1, suggesting that the analgesic effect of esketamine was limited to the early postoperative period. The postoperative analgesic effect may be related to its antagonism to NMDA receptors and the prevention of acute opioid tolerance and hyperalgesia [23,24,25]. Moreover, a recent meta analysis conducted by Xuemei Wang et al. [25] provided a moderate-to-low level of certainty that esketamine was associated with a significant reduction in pain intensity at 4, 12, and 24 h of rest after surgery and a decline in opioid demand at 4 and 12 h after surgery. However, our trial did not observe statistical significance in the amount of analgesia used on POD 1, as expected. According to our data collector’s remark, some patients in group C hesitated to use the analgesic for fear of side effects. This might underlie the two groups’ nonsignificant difference in 24-h dezocine consumption.
In addition, esketamine also has antidepressant effects [26, 27]. Alternative hypotheses include selectivity for the NMDA receptor subtype containing the NMDA receptor subunit 2B (NR2B), inhibition of the phosphorylation of the eukaryotic elongation factor 2 (eEF2) kinase, increased expression of brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrKB), and activation of the mammalian target of rapamycin (mTOR) signaling pathway, alongside other independent actions attributed to the esketamine [26]. The favorable results seen in the emotional state domain on POD 1 may be attributable to these mechanisms above. The effect of esketamine on emotion and pain might account for the enhanced postoperative quality of early recovery. However, we recognize that our study was underpowered to determine the difference in individual dimensions of QoR-40 questionnaire. Further studies are needed to confirm.
Volatile or intravenous anesthetics often reduce systemic vascular resistance and myocardial contractility, leading to decreased arterial pressure or cardiac output [15, 28,29,30,31]. On the contrary, esketamine excites the cardiovascular system through its sympathomimetic effects in a concentration-dependent manner [32, 33]. During anesthesia, the combined application of esketamine can compensate for the circulatory inhibition produced by other drugs [28, 34]. Ning Zhou et al. [28] found that esketamine produced higher MAP values than placebo before and after the skin incision. In our trial, while there was no statistical difference in heart rate and mean arterial pressure between the two groups, lower use of phenylephrine drugs in esketamine group was observed compared with in the control group. It means esketamine may enhance the patients’ hemodynamic profile. Our study excluded patients with grade III hypertension, severe arrhythmias, and unstable hemodynamic instability. Thus this conclusion does not apply to these patients.
Based on the results of our study, the Ai was significantly higher in the ketamine groups than in the control group. Similar results were found in other studies with bispectral index (BIS) monitoring. Hamp et al. noted that the BIS in the intravenous esketamine groups was roughly twice as high as in the placebo [35]. The mechanism is still unknown, but is presumed to result from the cerebral excitatory activity caused by esketamine [36]. Moreover, we found neither intraoperative awareness nor body movements in the two groups. These findings suggest that higher Ai or BIS scores are not equal to clinically inadequate sedation, while ketamine indeed had a relevant effect on depth of anesthesia judgment like Ai or BIS. Our sample size was insufficient to draw a definite conclusion on the relationship between the Ai value and level of sedation. More studies are needed. Anesthesiologists need to be cautious about interpreting BIS or Ai and take actual clinical symptoms into account when using esketamine.
Similar to previous trials, intraoperative esketamine was tolerated well in our study. There were no significant differences regarding the time of emergence, first out-of-bed, postoperative urinary retention, or length of hospital stay found between the groups. Neither PONV nor CNS adverse event rates were affected by esketamine. The insignificant result of PONV might be due to the systematic prophylaxis and multimodal analgesia in the perioperative period. Ketamine is limited in clinical use for its CNS adverse reactions [37]. In our study, minor CNS adverse event rates were found. On one hand, except for greater analgesic and anesthetic activity, esketamine has fewer psychotomimetic effects than the racemic mixture and R-isomer [38]. On the other, all patients were given midazolam at induction to prevent the neurotoxic effects of ketamine [39].
Our trial had several strengths, including allocation concealment, double-blinding, and a prospective design. Meanwhile, we also prepared emergency envelopes for unblinding and resuscitation if needed. Furthermore, all surgeries in the trial were performed by the same team to minimize confounding factors. In addition, we were the first to evaluate the effect of esketamine on postoperative recovery after bariatric surgery. Patients usually prioritize their subjective perception, feelings, and mood over objective examination data like laboratory values and imaging. The QoR-40 questionnaire provides a patient-centered measure of recovery quality, which holds more clinical significance for patients. Therefore, we used the QoR-40 questionnaire on POD 1 as our primary outcome. Our study suggests that esketamine could be a viable option for enhancing patients’ postoperative experience and recovery. Furthermore, this conclusion could provide new insights for anesthesia in obese patients undergoing other types of surgeries.
The study undeniably has its limitations. First, the plasma concentration of esketamine was not detected in the study. Second, the most effective administration and amount of esketamine remain controversial. Although our dosing regimen proved effective, it is likely to be sub-optimal. Third, although our study was strictly blind-controlled, hemodynamic and Ai changes may have introduced some potential bias. Fourth, the QoR-40 questionnaire is based on subjective experience. We ignored the latent learning effect caused by repeated uses of the QoR-40 questionnaire. And the sample size calculated for the total QoR-40 score was insufficient for determining differences between groups on individual dimensions. Finally, as a single-center study, the use of intravenous-inhalation combined anesthesia was a common practice at our hospital. Although this reflects the actual clinical situation, it may limit the generalizability to some extent. Future multicenter studies involving different anesthetic protocols are needed to further validate our results.
Conclusions
Continuous ketamine infusion seems to be safe and well tolerated in laparoscopic bariatric surgery. It improved the quality of postoperative recovery and reduced pain on POD 1. In spite of the increased Ai value during the surgery, it also provided better hemodynamics with decreased usage of phenylephrine. In the future, more comparative studies with varying doses of esketamine are needed to determine which methods can provide optimal postoperative recovery effects.
References
Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess (Winchester, England). 2009;13(41):1–190, 215–357, iii–iv.
Ogunnaike BO, Jones SB, Jones DB, Provost D, Whitten CW. Anesthetic considerations for bariatric surgery. Anesth Analg. 2002;95(6):1793–805.
Zhao J, Cai S, Zhang L, et al. Progress, challenges, and prospects of research on the effect of gene polymorphisms on adverse reactions to opioids. Pain Ther. 2022;11(2):395–409.
Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74(9):834–8.
Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19(6):370–80.
De Oliveira Jr GS, Fitzgerald PC, Hansen N, Ahmad S, McCarthy RJ. The effect of ketamine on hypoventilation during deep sedation with midazolam and propofol: a randomised, double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2014;31(12):654–62.
Eikermann M, Grosse-Sundrup M, Zaremba S, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116(1):35–46.
Hung KC, Wu SC, Chang PC, et al. Impact of intraoperative ketamine on postoperative analgesic requirement following bariatric surgery: a meta-analysis of randomized controlled trials. Obes Surg. 2021;31(12):5446–57.
Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69(1):23–8.
Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–77.
Zhu M, Xu S, Ju X, Wang S, Yu X. Effects of the different doses of esketamine on postoperative quality of recovery in patients undergoing modified radical mastectomy: a randomized, double-blind, controlled trial. Drug Des Dev Ther. 2022;16:4291–9.
Yu L, Zhou Q, Li W, et al. Effects of esketamine combined with ultrasound-guided pectoral nerve block type II on the quality of early postoperative recovery in patients undergoing a modified radical mastectomy for breast cancer: a randomized controlled trial. J Pain Res. 2022;15:3157–69.
Cheng X, Wang H, Diao M, Jiao H. Effect of S-ketamine on postoperative quality of recovery in patients undergoing video-assisted thoracic surgery. J Cardiothorac Vasc Anesth. 2022;36(8):3049–56.
Brinck ECV, Maisniemi K, Kankare J, et al. Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg. 2021;132(1):69–79.
Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401.
GuaráSobrinho H, Garcia JB, Vasconcelos JW, Sousa JC, Ferro LS. Analgesic efficacy of the intra-articular administration of S(+)-ketamine in patients undergoing total knee arthroplasty. Rev Bras Anestesiol. 2012;62(5):665–75.
Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Dev Ther. 2019;13:4135–44.
Eberl S, Koers L, van Hooft JE, et al. Sedation with propofol during ERCP: is the combination with esketamine more effective and safer than with alfentanil? Study protocol for a randomized controlled trial. Trials. 2017;18(1):472.
Chen J, Zou X, Hu B, et al. Effect of different doses of esketamine compared with fentanyl combined with propofol on hypotension in patients undergoing painless abortion surgery: a prospective, randomized, double-blind controlled clinical trial. BMC Anesthesiol. 2022;22(1):305.
Zhu T, Zhao X, Sun M, et al. Opioid-reduced anesthesia based on esketamine in gynecological day surgery: a randomized double-blind controlled study. BMC Anesthesiol. 2022;22(1):354.
Miziara LE, Simoni RF, Esteves LO, et al. Efficacy of continuous S(+)-ketamine infusion for postoperative pain control: a randomized placebo-controlled trial. Anesthesiol Res Pract. 2016;2016:6918327.
Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125(1):39–45.
Ilkjaer S, Dirks J, Brennum J, Wernberg M, Dahl JB. Effect of systemic N-methyl-d-aspartate receptor antagonist (dextromethorphan) on primary and secondary hyperalgesia in humans. Br J Anaesth. 1997;79(5):600–5.
Kissin I, Bright CA, Bradley EL Jr. The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg. 2000;91(6):1483–8.
Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: a systematic review and meta-analysis. J Clin Anesth. 2021;68: 110071.
Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411–20.
Liu P, Li P, Li Q, et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Investig Surg. 2021;34(8):883–8.
Zhou N, Liang X, Gong J, et al. S-ketamine used during anesthesia induction increases the perfusion index and mean arterial pressure after induction: a randomized, double-blind, placebo-controlled trial. Eur J Pharm Sci. 2022;179: 106312.
Goodchild CS, Serrao JM. Propofol-induced cardiovascular depression: science and art. Br J Anaesth. 2015;115(4):641–2.
Lowe D, Hettrick DA, Pagel PS, Warltier DC. Influence of volatile anesthetics on left ventricular afterload in vivo. Differences between desflurane and sevoflurane. Anesthesiology. 1996;85(1):112–20.
Degoute CS, Ray MJ, Manchon M, Dubreuil C, Banssillon V. Remifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplasty. Can J Anaesth. 2001;48(1):20–7.
Sigtermans M, Dahan A, Mooren R, et al. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic–pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111(4):892–903.
Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9–10):356–66.
Kasputytė G, Karbonskienė A, Macas A, Maleckas A. Role of ketamine in multimodal analgesia protocol for bariatric surgery. Medicina (Kaunas). 2020;56(3):96.
Hamp T, Baron-Stefaniak J, Krammel M, et al. Effect of intravenous S-ketamine on the MAC of sevoflurane: a randomised, placebo-controlled, double-blinded clinical trial. Br J Anaesth. 2018;121(6):1242–8.
Matsushita S, Oda S, Otaki K, Nakane M, Kawamae K. Change in auditory evoked potential index and bispectral index during induction of anesthesia with anesthetic drugs. J Clin Monit Comput. 2015;29(5):621–6.
Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19(9):1033–46.
Muller J, Pentyala S, Dilger J, Pentyala S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6(3):185–92.
Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12(12): CD012033.
Acknowledgements
The authors would like to acknowledge the support of patients who have generously given their time to complete the questionnaires for the research protocol.
Funding
This research was funded by the National Natural Science Foundation of China [grant number 82270059], China Primary Health Care Foundation [grant number YLGX-MZ-2022004], Jiangsu Provincial Health Commission Medical Research Key Project [grant number K2019003], and Jiangsu Natural Science Foundation [grant number BK20221222]. No funding or sponsorship was received for this study or publication of this article. The rapid service fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Authorship Contributions
Conceptualization, writing, review, and editing were performed by Jingyue Zhang. Design of the study, methodology, and writing were carried out by Jingjing Dang. Investigation and acquisition of data were performed by Huiwen Zheng and Ronghua Zuo. Statistical analysis and writing were performed by Chao Liu and Baiqing Ren. Data curation, supervision, and reviewing were performed by Rui Wang and Tianya Liu. Review and editing were performed by Fan Wang. Conceptualization, provision of critical comments, review and editing, and project administration were performed by Zhiping Wang. All authors have read and agreed to the published version of the manuscript.
Disclosures
All named authors have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the ethics of committee of Xuzhou Medical University Affiliated Hospital (XYFY2022-KL037-02) and registered at the Chinese Clinical Trial Registry (ChiCTR220006613). Written informed consent was obtained from all subjects involved in the study. The study was conducted in accordance with the Declaration of Helsinki and its later amendments.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, J., Wang, F., Dang, J. et al. Effect of Intraoperative Infusion of Esketamine on Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery: A Randomized Controlled Trial. Pain Ther 12, 979–992 (2023). https://doi.org/10.1007/s40122-023-00519-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00519-9