Abstract
Introduction
Numerous medications are used for the preventive treatment of chronic migraine (CM), including oral treatments, onabotulinumtoxinA (onabotA; BOTOX), and calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs). Despite substantial clinical trial evidence, less is published about the real-world experience of these treatments based on data routinely collected from a variety of sources. This systematic review assessed real-world evidence on the effectiveness and safety of preventive treatments for CM in adults.
Methods
A systematic search of MEDLINE, Embase, and the Cochrane library with back-referencing and supplementary searches retrieved data published between January 2010 and February 2020. Publications were screened, extracted, and quality assessed. Data were narratively synthesized. Search criteria included preventive medications for CM. Evidence was available for topiramate, onabotulinumtoxinA, CGRP mAbs (erenumab, galcanezumab, and fremanezumab). OnabotulinumtoxinA was most commonly assessed (55 studies), followed by erenumab (six studies), multiple CGRP mAbs (one study), and topiramate (one study). Long-term data (> 1 year) were available for onabotulinumtoxinA only, with erenumab reported up 6 months, topiramate up to 3 months, and multiple CGRP mAbs up to 12 months.
Results
Substantial data demonstrated that onabotulinumtoxinA reduces the number/frequency of headaches, concomitant acute medication use, and impact of headaches on well-being and daily activity. More limited evidence showed benefits for the same parameters with erenumab. Single studies suggested topiramate and multiple CGRP mAbs decrease the number/frequency of headaches and impact of headaches. To date, onabotulinumtoxinA is the only preventive treatment for CM that has long-term safety data in real-world settings reporting treatment-related adverse events of up to 3 years.
Conclusion
While substantial real-world evidence supports the long-term effectiveness and safety of onabotulinumtoxinA, real-world data on other preventive treatments of CM are currently limited to short term effectiveness due to their more recent approvals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic literature review identified evidence from real-world studies for the preventive treatment of chronic migraine (CM). |
Thirty-eight studies met the selection criteria and reported monthly headache or migraine day data: onabotulinumtoxinA (n = 34), erenumab (n = 4), topiramate versus onabotulinumtoxinA (n = 1), and erenumab, galcanezumab, and fremanezumab versus onabotulinumtoxinA (n = 1). |
The reduction from baseline in monthly headache and migraine days at all time points was statistically significant with all interventions. |
OnabotulinumtoxinA had the largest number of studies with the longest follow-up periods, and produced a beneficial effect on the number and frequency of headache or migraine days, concomitant medication use, and impact of headache [i.e., Headache Impact Test-6 (HIT-6)]. |
No new safety concerns were observed with long-term use of any of these treatments. |
Introduction
Chronic migraine (CM) is defined by the International Headache Society Classification of Headache Disorders (ICHD-3) as a headache occurring on at least 15 days per month for more than 3 months, with migraine on at least 8 days per month [1]. These eight migraine days can be defined by migraine features, aura, or response to migraine-specific treatment. CM can be debilitating for many patients, with negative effects on physical, social, and occupational functioning, and a reduced health-related quality of life [2, 3]. Overall, global CM prevalence is 1–2% [4].
Management of CM is focused on preventive treatment to reduce headache frequency and severity, and to limit reliance on acute treatment. Numerous oral agents are used for the preventive treatment of CM, including antihypertensives (beta-blockers, calcium-channel blockers), antidepressants (tricyclic antidepressants, selective serotonin reuptake inhibitors, noradrenergic and specific serotonergic antidepressants), and anticonvulsants [2]. However, there tends to be limited evidence for their use in CM, and treatments are often discontinued due to lack of efficacy or poor tolerability [2, 5,6,7].
High-quality clinical trial evidence is available for the use of topiramate, onabotulinumtoxinA (OnabotA; BOTOX), and calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) in CM [2]. OnabotulinumtoxinA was approved in the European Union (EU) for symptom relief in chronic migraine and the USA for prophylaxis of headaches in adult patients with CM in 2010 [8], and has long-term, real-world evidence in a wide patient population [2, 8, 9]. The broad-spectrum antiepileptic drug, topiramate, has been approved for migraine prevention in adults in Europe since 2003 and in the USA since 2004 [10], while CGRP mAbs (erenumab, galcanezumab, fremanezumab, and eptinezumab) have been approved since 2018 [11, 12].
While clinical trial evidence exists for topiramate, onabotulinumtoxinA, and CGRP mAbs, less is known about the comparative real-world effectiveness and safety of these treatments. We conducted a systematic literature review (SLR) to assess and summarize the real-world evidence on the effectiveness and safety of all pharmacological agents used for preventive treatment of CM in adults. As the literature review specifically focuses on real-world studies, the term “effectiveness” (used to describe the degree of beneficial effect including tolerability under “real-world” clinical settings) rather than “efficacy” (defined as the benefit of treatment under ideal conditions) is used throughout [13].
Methods
This SLR was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines [14]. A PRISMA 2009 Checklist is provided as Supplementary file S1.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
Searches were performed in Medical Literature Analysis and Retrieval System Online (MEDLINE), the Excerpta Medica database (Embase) via embase.com, and the Cochrane library/Cochrane Central Register of Controlled Trials via Cochrane library to identify relevant real-world data published in any language between 1 January 2010 and 11 February 2020. Each search was conducted using controlled vocabulary and keywords (“migraine” OR “migrainous headache” OR “hemicrania” OR “status hemicranicus” OR “chronic migraine”), and was limited to prospective cohort studies, retrospective cohort studies, cross-sectional studies, and case–control studies involving at least 40 human adult subjects with a reported duration of follow-up. Clinical trials, case series, and case reports were excluded. A summary of the review protocol is provided as Supplementary file S1.
A systematic search approach was used, in which specific disease and study design facets were developed for the embase.com and Cochrane library search strategies. The disease facet was combined with the study design facet using “AND“ as the Boolean operator (Supplementary file S3).
In addition to the structured searches, supplementary searches were also conducted to ensure all relevant data were retrieved. Supplementary searches included hand searching of relevant conferences between January 2017 and March 2020, bibliographic searches of systematic reviews and narrative reviews published in the last 3 years, and manual searches of PubMed, Google, and Google Scholar. Trial registry searches (Clinicaltrials.gov and the EU Clinical Trials Register) were also conducted to retrieve a list of ongoing and planned real-world observational studies for the relevant population.
Selection Criteria
Study selection was undertaken in two steps. Initially, the title and abstract (ti/ab) of each citation was screened to identify a list of potentially relevant studies, then the full-text versions of relevant studies were reviewed to determine the final list of included studies. For both the ti/ab and full-text screening, all citations were included or excluded on the basis of a screening flowchart (Supplementary file S4). The studies that met all predefined criteria for inclusion were included for final extraction, while excluded citations were coded by reason for exclusion.
Data Extraction
Study characteristics, patient characteristics, treatment details (name of intervention, dose, duration, and frequency), as well as effectiveness and safety outcomes [treatment-related adverse events (TRAEs)] were extracted into a Microsoft Excel data extraction sheet.
Quality Assessment
A quality assessment of each full journal publication included in the review was carried out using the Newcastle–Ottawa scale (NOS), in which assessment criteria vary according to study type (cohort studies or case–control studies) [15]. Details are provided as Supplementary file S5.
Results
Study and Patient Characteristics
The PRISMA flow diagram of included studies is shown in Fig. 1. Database searches detected 12,662 potentially relevant citations, with 11 further citations identified through supplementary searches. After removal of duplicates, 382 relevant records were selected through ti/ab screening. Following a detailed examination of the full-text articles, a total of 130 publications comprising 61 primary studies (34 journal publications and 27 conference abstracts) were included for data extraction.
PRISMA flow diagram for included studies. Because a given study may be reported in more than one publication, we have defined a primary publication as the first full report of the primary outcomes of a study in a peer-reviewed journal. Additional reports of secondary or exploratory objectives, or subgroup or post-hoc analyses are described as “secondary” or “linked” publications
A complete list of included studies along with any linked publications is provided in Supplementary file S6.
Study Characteristics
The disposition of the studies according to key parameters is provided in Table 1. In total, there were 39 prospective cohort studies and 22 retrospective cohort studies. The sample size in the included studies ranged from 42 [16] to 1160 [17]. The 34 journal publications for which quality assessment was conducted were of moderate-to-high quality, and had a score of ≥ 4 on NOS. Studies reported as conference abstracts only were not eligible for quality assessment (Table 2).
Among the 61 studies, most (n = 55) assessed the effectiveness or safety of onabotulinumtoxinA. Other studies assessed erenumab (n = 6), multiple CGRP mAbs (n = 1), and topiramate (n = 1). No studies assessing the effectiveness or safety of flunarizine, sodium valproate, acetylsalicylic acid, amitriptyline, or any other toxin were identified.
Thirty-nine studies directly assessed patients in hospitals or clinics (primary data), while data were taken from medical records or databases in 18 studies. Other data sources included surveys (n = 2) and registries (n = 1). Duration of follow-up across included studies ranged from 1 month [18, 19] to 8 years (Hull Migraine study [20]). Forty-two studies were conducted in Europe, 12 in North America, two in Asia, two in Australia, and two were conducted across multiple countries. The country where the study was conducted was not reported in one case. All the included studies reported effectiveness of the assessed intervention, while TRAEs were reported in only six studies.
Patient Characteristics
Patient characteristics are summarized in Table 3. The mean or median age of participating patients was reported in 43 studies, and ranged from 39.3 years [21] to 54.0 years [22]. Among the 43 studies that reported sex, most patients (71.0% [16] to 97.9% [23]) were female. The mean/median disorder duration ranged from 1.5 years [23] to 33.6 years [24].
The most commonly reported comorbidities among the nine studies with relevant data were anxiety and depression. The proportion of patients receiving prior preventive treatment ranged from 21.0% [25] to 93.0% [26]. Although prior use of preventive medications was reported in 37 studies, no studies were conducted in a purely treatment-naïve population, and no studies conducted subgroup analyses comparing effectiveness or safety outcomes among treatment-naïve patients and those with prior preventive treatment failures. Table 4 provides studies by treatment and prior preventive treatment status.
Effectiveness Outcomes
Effectiveness outcomes assessed in the included studies are summarized in Table 5. The outcomes most commonly assessed were response rate (reduction in headache or migraine days), monthly headache or migraine days, concomitant acute medications used, and Headache Impact Test-6 (HIT-6 score).
Response Rates
Response rates were defined as the reduction in the number of headache or migraine days over a specified time period. The definition of headache day varied across studies, but for the purposes of this study was defined as any day with a headache lasting at least 30 min. A migraine day was defined as any day with headache with migraine features such as photophobia, phonophobia, nausea, vomiting, or worsening with physical activity. Response rates were quantified according to the proportion of patients with ≥ 30% (16 studies), ≥ 50% (30 studies), or ≥ 75% (six studies) reduction in headache or migraine days from baseline at different time points.
Thirty-six studies reported data for response rates. Substantially more data on response rates were available for onabotulinumtoxinA (n = 32 studies), compared with erenumab (four studies) and multiple CGRP mAbs (one study). Follow-up data beyond 1 year were available for onabotulinumtoxinA only (up to 4 years), with 1-year data available for multiple CGRP mABs and 6-month data available for erenumab.
Response rate data across all included studies are summarized in Table 6. All studies reported a reduction in number of headache or migraine days, irrespective of treatment used, at all time points. One comparative study reported as an abstract only [16] reported a higher proportion of patients with ≥ 50% reduction in migraine days at 1 year with multiple CGRP mAbs than with onabotulinumtoxinA (62.0% versus 52.0%); although this difference was not statistically significant (quality rating N/A).
Detailed summaries of response rates across included studies are presented in Supplementary file S7.
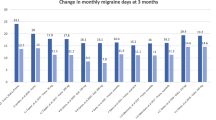
Monthly Headache or Monthly Migraine Days
Monthly headache days were defined as the number of days in any given month on which a headache lasting at least 30 min was reported. Monthly migraine days were defined as the number of days in any given month on which a headache with migraine features such as photophobia, phonophobia, nausea, vomiting, or worsening with physical activity was reported.
Thirty-eight studies reported data for monthly headache or migraine days. Most studies (n = 34) assessed onabotulinumtoxinA and four assessed erenumab, while one study assessed monthly headache days in topiramate compared with onabotulinumtoxinA, and one study assessed monthly migraine days in multiple CGRP mAbs (erenumab, galcanezumab, and fremanezumab) in comparison with onabotulinumtoxinA. At up to 5 years, onabotulinumtoxinA had the longest follow-up data on monthly headache or monthly migraine days, compared with 6 months for erenumab, 12 months for multiple CGRP mAbs, and 3 months for topiramate.
Mean/median monthly headache days and monthly migraine days, and change from baseline, at different time points across included studies are summarized in Table 7. The reduction from baseline in monthly headache days and monthly migraine days at all time points was statistically significant with all interventions. A single study [27] comparing onabotulinumtoxinA with topiramate (NOS quality rating, 5/9) reported that patients treated with onabotulinumtoxinA had significantly fewer monthly headache days compared with topiramate at 3 months (13.8 days versus 14.9 days, p < 0.05).
Detailed summaries of monthly headache and migraine days across included studies are presented in Supplementary file S8.
Concomitant Acute Medication Use
Concomitant acute medication use was defined as the usage of opioid and non-opioid analgesics for acute relief of migraine during the study period.
Twenty-six studies reported data for concomitant acute medication use. A large majority (n = 24) assessed onabotulinumtoxinA, and only two assessed erenumab. OnabotulinumtoxinA had the longest follow-up data on concomitant medication use (5 years), compared with 3 months for erenumab. No comparative studies were identified for concomitant acute medication use.
Mean/median days per month with concomitant acute medication use and change from baseline at different time points among the included studies are summarized in Table 8. Reduction in concomitant acute medication use from baseline was statistically significant at all time points in most studies.
Detailed summaries of acute medication use across included studies are presented in Supplementary file S9.
Headache Impact Test-6 (HIT-6) Score
HIT-6 score evaluates the effects of headache on general patient well-being and daily activity. The score ranges from 36 to 78, with a higher score indicating a lower level of general well-being and daily activity. The four headache impact severity categories are little or no impact (49 or less), some impact (50–55), substantial impact (56–59), and severe impact (60–78).
Eighteen studies reported data for HIT-6 scores. Of these, 16 assessed onabotulinumtoxinA, two assessed erenumab, and one study each assessed multiple CGRP mAbs and topiramate (in comparison with onabotulinumtoxinA). At up to 3 years, onabotulinumtoxinA had the longest follow-up data on monthly headache or monthly migraine days, compared with 3 months for erenumab, 12 months for multiple CGRP mAbs, and 3 months for topiramate.
Mean/median HIT-6 scores and change from baseline at different time points across the included studies are summarized in Table 9. Reduction in HIT-6 score from baseline was statistically significant at all time points for all interventions. The single study comparing onabotulinumtoxinA with topiramate (NOS quality rating, 5/9) reported that patients treated with onabotulinumtoxinA had a significantly lower score on the HIT-6 scale compared with topiramate at 3 months (39.5 versus 43.2; p < 0.05) [27]. The abstract comparing onabotulinumtoxinA and multiple CGRP mAbs (NOS quality rating, N/A) showed that 35.0% of those treated with onabotulinumtoxinA and 44.0% of those treated with multiple CGRP mAbs experienced at least one severity class improvement according to HIT-6 score at 1 year; this difference was not significant [16].
Detailed summaries of HIT-6 score use across included studies are presented in Supplementary file S10.
Safety Outcomes (TRAEs)
Six studies reported data on TRAEs. The duration of these studies ranged from 10 to 36 months, and all evaluated patients were treated with onabotulinumtoxinA.
In four studies with a follow-up of 1 year, the proportion of patients experiencing at least one TRAE ranged from 0% to 25.1%. One study with a follow-up of 2 years (NOS quality rating, 6/9) reported that 17.5–20.3% of patients experienced at least one TRAE [28], and one study with a follow-up of 3 years (NOS quality rating, 5/9) reported at least one TRAE in 20% of patients [29].
Discussion
This SLR summarizes the real-world evidence available on the effectiveness and safety of preventive treatments for CM in adults. Data were retrieved for onabotulinumtoxinA, CGRP mAbs (erenumab, galcanezumab, and fremanezumab), and topiramate. No studies reporting the effectiveness or safety of flunarizine, sodium valproate, acetylsalicylic acid, amitriptyline, or any other toxin in the preventive treatment of CM were identified within the parameters of this review.
The overwhelming majority of real-world data was reported on onabotulinumtoxinA, with 55 published studies identified. Six studies reported on erenumab, one study reported on multiple CGRP mAbs (erenumab, galcanezumab, and fremanezumab), and one study reported on topiramate. Long-term data (up to 8 years) were available for onabotulinumtoxinA only, with data for multiple CGRP mAbs up to 12 months, erenumab reported up to 6 months, and topiramate up to 3 months. The greater availability of onabotulinumtoxinA data is probably because onabotulinumtoxinA has been approved for CM treatment since 2010, while the data for other interventions are limited due to their more recent approval.
A considerable body of evidence demonstrates that onabotulinumtoxinA is effective in reducing both the number and frequency of headaches among adults with CM, and also reduces the need for use of concomitant acute medication. In addition, numerous studies reported a reduced impact of headache on patient well-being and daily activity, as assessed by the HIT-6 score. Up to 25% of patients receiving onabotulinumtoxinA in the included studies experienced at least one TRAE, but no new long-term safety concerns were identified.
Similarly, erenumab was also shown to reduce the number and frequency of headaches and concomitant acute medication use, and to decrease the impact of headaches on well-being and daily activity. Single studies suggested topiramate and multiple CGRP mAbs decrease the number/frequency of headaches and impact of headaches. To date, onabotulinumtoxinA is the only preventive treatment for CM that has long-term safety data reporting TRAEs of up to 3 years.
Owing to the lack of high-quality comparative real-world studies, it is not possible to comment on the relative effectiveness of the treatments included in this review. However, the availability of a considerable body of real-world data from a large patient population, encompassing different patient nationalities, ages, comorbidities, and previous prior preventive treatments, provides consistent and predictable evidence for the benefits of onabotulinumtoxinA as a preventive treatment for CM. This body of evidence, as also described in a recently published meta-analysis [30], supports the effectiveness and safety of this approach in the management of CM.
Although this was a comprehensive SLR conducted according to PRISMA guidelines, a number of limitations should be highlighted. The paucity of data identified for treatments other than onabotulinumtoxinA is a significant limitation, precluding any robust analysis of alternative treatments. Comparisons across treatments are also difficult to establish given the lack of data for treatments other than onabotulinumtoxinA, and because only one publication and one abstract (NOS quality rating 5/9 and N/A, respectively) included a direct comparison of two or more interventions. It is also important to note that the patient populations included across studies are inevitably heterogeneous, and any comparisons of data across multiple studies should therefore be interpreted with caution.
Since the regulatory approval of the CGRP monoclonal antibodies in 2018, numerous additional studies are now being published on these treatments. These publications are not included in this analysis as, for practical considerations, a cut-off date for data collection needed to be established prior to drafting the manuscript. Future analyses will be considered to include more recently approved interventions.
Conclusions
This SLR identified substantial evidence from real-world studies for onabotulinumtoxinA in the preventive treatment of CM. The review suggests that treatment with onabotulinumtoxinA has a beneficial effect across a broad range of patients in real-world clinical studies on the number and frequency of headache or migraine days, concomitant medication use, and HIT-6. Additionally, no new safety concerns were observed with long-term use. Real-world data were available from conference abstracts only for erenumab and multiple CGRP mAbs (erenumab, galcanezumab, and fremanezumab), and one journal article on topiramate. Although the data in these treatments are more limited, there is some evidence of the effectiveness of these approaches in the real world.
References
International Headache Society. 2019 [cited 2020 12 September]. https://ichd-3.org/1-migraine/1-3-chronic-migraine/. Accessed 12 Sep 2022.
Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92.
Adams AM, Serrano D, Buse DC, et al. The impact of chronic migraine: the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35:563–78.
Burch R, Hettie B. Longitudinal preventive medication use patterns in patients receiving onabotulinumtoxina treatment: a chart review study. Headache. 2019;59:41–2.
Ford JH, Jackson J, Milligan G, Cotton S, Ahl J, Aurora SK. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. 2017;57:1532–44.
Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20:22–33.
Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478–88.
Frampton JE, Silberstein S. OnabotulinumtoxinA: a review in the prevention of chronic migraine. Drugs. 2018;78:589–600.
Gooriah R, Ahmed F. OnabotulinumtoxinA for chronic migraine: a critical appraisal. Ther Clin Risk Manag. 2015;11:1003–13.
Le K, Yu D, Wang J, Ali AI, Guo Y. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:6.
Spindler BL, Ryan M. Medications approved for preventing migraine headaches. Am J Med. 2020;133:664–7.
Rothrock JF, Adams AM, Lipton RB, et al. FORWARD Study: evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache. 2019;59:1700–13.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, et al,. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013 [cited 2020 11 September ]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 11 Sep 2022.
Taddei-Allen P, Deleon L, Oelofsen M, Page N. Comparing patient-reported outcomes in patients using CGRP antagonists or onabotulinumtoxinA for chronic migraine. J Manag Care Spec Pharm. 2019;25:S60.
Matharu M, Pascual J, Nilsson R, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37:1384–97.
Garcia-Azorin D, Ruiz-Pinero M, Sierra A, Martinez B, Guerrero Peral AL. Habituation determined by algometry as a marker of response in chronic migraine. J Headache Pain. 2018;19(Suppl 1):80. Abstract P154.
Lee MJ, Lee C, Choi H, Chung C. Factors associated with favorable outcome in botulinum toxin A treatment for chronic migraine: a clinic-based prospective study. J Neurol Sci. 2016;363:51–4.
Ahmed F, Zafar HW, Buture A, Khalil M. Does analgesic overuse matter? Response to OnabotulinumtoxinA in patients with chronic migraine with or without medication overuse. Springerplus. 2015;4:589.
Aydinlar EI, Dikmen PY, Kosak S, Kocaman AS. OnabotulinumtoxinA effectiveness on chronic migraine, negative emotional states and sleep quality: a single-center prospective cohort study. J Headache Pain. 2017;18:23.
Flood P, Barad M, Sturgeon J, Kao M, Fish S, Mackey S. PREEMPT: Prospective from complex patients in practice. J Pain. 2017;18:S67.
Domínguez C, Vieites-Prado A, Pérez-Mato M, et al. CGRP and PTX3 as predictors of efficacy of Onabotulinumtoxin type a in chronic migraine: an observational study. Headache. 2018;58:78–87.
Russo M, Manzoni GC, Taga A, et al. The use of onabotulinum toxin A (Botox®) in the treatment of chronic migraine at the Parma Headache Centre: a prospective observational study. Neurol Sci. 2016;37:1127–31.
Schiano di Cola F, Caratozzolo S, Liberini P, Rao R, Padovani A. Response predictors in chronic migraine: Medication overuse and depressive symptoms negatively impact onabotulinumtoxin-A treatment. Front Neurol. 2019;10:678.
Aicua-Rapun I, Martínez-Velasco E, Rojo A, et al. Real-life data in 115 chronic migraine patients treated with Onabotulinumtoxin A during more than one year. J Headache Pain. 2016;17:112.
Naprienko MV, Smekalkina LV, Safonov MI, et al. The burden of migraine in real clinical practice: clinical and economic aspects. Neurosci Behav Physiol. 2020;50:20–6.
Negro A, Curto M, Lionetto L, Martelletti P. A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. 2016;17:1–9.
Guerzoni S PL, Baraldi C, Cainazzo MM, Negro A, Martelletti P, Pini LA. Long-term treatment benefits and prolonged efficacy of OnabotulinumtoxinA in patients affected by chronic migraine and medication overuse headache over 3 years of therapy. Front Neurol. 2017;8:586.
Lanteri-Minet M, Ducros A, Francois C, Olewinska E, Nikodem M, Dupont-Benjamin L. Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: a meta-analysis on 10 years of real-world data. Cephalalgia. 2022. https://doi.org/10.1177/03331024221123058.
Ahmed F, Gaul C, García-Moncó JC, Sommer K, Martelletti P. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20:26.
Alessiani M, Petolicchio B, Vigano A, et al. Short-term psychodynamic psychotherapy versus onabotulinumtoxinA as preventive therapy in chronic migraine: a real world study. J Headache Pain. 2018;19(Suppl 1):80. Abstract O50.
Alpuente A, Gallardo VJ, Torres-Ferrus M, Alvarez-Sabin J, Pozo-Rosich P. Early efficacy and late gain in chronic and high-frequency episodic migraine with onabotulinumtoxinA. Eur J Neurol. 2019;26:1464–70.
Andreou AP, Trimboli M, Al-Kaisy A, et al. Prospective real-world analysis of OnabotulinumtoxinA in chronic migraine post-National Institute for Health and Care Excellence UK technology appraisal. Eur J Neurol. 2018;25:1069–75.
Barad M, Sturgeon JA, Fish S, Dexter F, Mackey S, Flood PD. Response to BotulinumtoxinA in a migraine cohort with multiple comorbidities and widespread pain. Reg Anesth Pain Med. 2019;44:660–8.
Barbanti P, Aurilia C, Egeo G, Fofi L, Vernieri F. Erenumab in migraine: the first Italian real-life data. Cephalalgia. 2019;39:262–3.
Belvís R, Morollón N, Guasch M, Marrero P, Azahara A, Roig C. Effectiveness of OnabotulinumtoxinA in chronic migraine. If early introduction: faster, cheaper and more satisfactory. J Headache Pain. 2018;18:P98-P.
Boudreau G, Becker WJ, Graboski C, et al. Impact of onabotulinumtoxina on quality of life, health resource utilization, and work productivity in people with chronic migraine: interim results from a prospective, observational study (PREDICT). Headache. 2018;58:94.
Boudreau GP, Demers C. Treatment of chronic migraine with Erenumab alone or as an add on therapy; a real world prospective observational study. Cephalalgia. 2019;39:367.
Butera C, Colombo B, Bianchi F, et al. Refractory chronic migraine: is drug withdrawal necessary before starting a therapy with onabotulinum toxin type A? Neurol Sci. 2016;37:1701–6.
Caronna E, Gallardo VJ, Hernández-Beltrán N, Torres-Ferrus M, Pozo-Rosich P. OnabotulinumtoxinA: an effective tool in the therapeutic arsenal for chronic migraine with medication overuse. Front Neurol. 2018;9:808.
Cernuda-Morollón E, Ramón C, Larrosa D, Alvarez R, Riesco N, Pascual J. Long-term experience with onabotulinumtoxinA in the treatment of chronic migraine: what happens after one year? Cephalalgia. 2015;35:864–8.
Cesaretti C, Molesti E, Lolli F, Amantini A, Lori S. O034. Type of pain and onabotulinumtoxin-A in chronic migraine: four years of follow-up. J Headache Pain. 2015;16:1–2.
Ching J, Tinsley A, Rothrock J. Prognosis following discontinuation of onabotulinumA therapy in “super-responding” chronic migraine patients. Headache. 2019;59:1279–85.
Corbelli I, Bernetti L, Verzina A, et al. Sustained efficacy and safety of onabotulinumtoxin Type A in chronic migraine patients: a multicentric prospective real-life study. Neurol Sci. 2019;40:S248.
de Tommaso M, Brighina F, Delussi M. Effects of botulinum toxin A on allodynia in chronic migraine: an observational open-label two-year study. Eur Neurol. 2019;81:37–46.
Domínguez C, Pozo-Rosich P, Torres-Ferrús M, et al. OnabotulinumtoxinA in chronic migraine: predictors of response. A prospective multicentre descriptive study. Eur J Neurol. 2018;25:411–6.
Evangelista L, Guezoni S, Pellesi L, et al. The profile of super-responders to onabotulinumtoxin A for chronic migraine: data from an observational study. J Headache Pain. 2017;18(Suppl 1):111. Abstract P137..
Favoni V, Calabro C, Asioli GM, et al. Can onabotulinumtoxina be successfully stopped in chronic migraine responders? Cephalalgia. 2019;39:242.
Forbes R, Kinney M, Singhal M, Forbes DL, Sugrue J. Treating chronic migraine with botulinum toxin Type A (BTX-A)-do affective disorders influence 12 month outcome? Cephalalgia. 2015;35:59.
Gomez-Galvan JB. Differences in response to onabotulinumtoxin a treatment according to the headache days suffered per month. Cephalalgia. 2016;36:20–1.
Gonzalez-Martinez A, Rodriguez Vazquez E, de la Red Gallego H, et al. Association between personality traits and onabotulinumtoxin A response in patients with chronic migraine. Headache. 2020;60:153–61.
Grazzi L, Usai S. Onabotulinum toxin A (Botox) for chronic migraine treatment: an Italian experience. Neurol Sci. 2015;36:33–5.
Grazzi L, D’Amico D, Raggi A, Leonardi M, Andrasik F. Botulinum a toxin for treatment of chronic migraine with medication overuse. Cephalalgia. 2016;36:21–2.
Jenkins B, Cheng S, Limberg N, Hutton E. Will refractory migraine patients in the real world respond to Erenumab? Cephalalgia. 2019;39:265–6.
Jimenez SR, Wilson MC. Safety and efficacy of botulinum toxin for chronic migraine in the elderly. Cephalalgia. 2015;35:53.
Kennedy G, Nightingale H, Richardson S. Longer term outcomes for patients with chronic migraine treated with OnabotulinumtoxinA BOTOX and implications for a Headache Service: Real-life data for 120 patients treated at Sunderland Royal Hospital, UK. Cephalalgia. 2017;37:333.
Lambru G, Trimboli M, Andreou AP, Murphy M, Al-Kaisy A. Long-term experience of onabotulinumtoxina in a large series of chronic migraine patients. Cephalalgia. 2016;36:47.
Lin K, Chen S, Fuh J, Wang Y, Wang S. Efficacy, safety, and predictors of response to botulinum toxin type A in refractory chronic migraine: a retrospective study. J Chinese Med Assoc. 2014;77:10–5.
Maasumi K, Thompson NR, Kriegler JS, Tepper SJ. Effect of onabotulinumtoxinA injection on depression in chronic migraine. Headache. 2015;55:1218–24.
Naegel S, Scheffler A, Wurthmann SF, Holle D. Erenumab for migraine treatment – first real world data from Germany. Cephalalgia. 2019;39:409.
Navarrete Perez JJ, Ruiz Piñero M, Gómez López de San Román C, et al. Wearing-off effect of onabotulinumtoxina in chronic migraine: evaluation in a series of 117 patients. Eur J Neurol. 2017;24:547.
Pedraza MI, de la Cruz C, Ruiz M, et al. OnabotulinumtoxinA treatment for chronic migraine: experience in 52 patients treated with the PREEMPT paradigm. SpringerPlus. 2015;4:176.
Ramirez AC, Alvarez FI, Volcy M. Efficacy of botulinum toxin type a botoxA® for chronic migraine, results from a headache center in Medellin- Colombia. Cephalalgia. 2013;33:257.
Ranoux D, Martiné G, Espagne-Dubreuilh G, Amilhaud-Bordier M, Caire F, Magy L. OnabotulinumtoxinA injections in chronic migraine, targeted to sites of pericranial myofascial pain: an observational, open label, real-life cohort study. J Headache Pain. 2017;18:75.
Robbins L. CGRP antagonists for chronic migraine: early results. Cephalalgia. 2019;39:231.
Robblee J, Mendez N, Potter J, Slonaker J, Starling AJ. Post-market observational study of patient experience with erenumab. Cephalalgia. 2019;39:258–9.
Santoro A, Tanzi M, Miscio A, Copetti M, Leone M. P025. Two-year follow-up with OnabotulinumtoxinA for chronic migraine: a real life evaluation of 113 patients. J Headache Pain. 2015;16(Suppl 1):A182.
Santoro A, Copetti M, Miscio AM, Leone MA, Fontana A. Chronic migraine long-term regular treatment with onabotulinumtoxinA: a retrospective real-life observational study up to 4 years of therapy. Neurol Sci. 2020;41(7):1809–20.
Sanz AC, Sánchez-Mateos NM, Hernández CS, et al. Experience with botulinum toxin in chronic migraine. Neurologia. 2018;33:499–504.
Sarchielli P, Romoli M, Corbelli I, et al. Stopping onabotulinum treatment after the first two cycles might not be justified: results of a real-life monocentric prospective study in chronic migraine. Front Neurol. 2017;8:655.
Stark C, Stark R, Limberg N, et al. Real-world effectiveness of onabotulinumtoxinA treatment for the prevention of headaches in adults with chronic migraine in Australia: a retrospective study. J Headache Pain. 2019;20(1):81.
Susie L, Maha A, Sarah M, Manjit M. Headache outcome measures in medically refractory chronic migraine patients treated with OnabotulinumtoxinA. J Headache Pain. 2018;19(Suppl 1):80. Abstract 025.
Velasco-Juanes F, Gómez-Esteban JC, Fernández-Valle T, et al. Clinical treatment of chronic and episodic migraine with onabotulinumtoxinA in a real-world setting. Drugs Ther Perspect. 2018;34:335–43.
Vernieri F, Paolucci M, Altamura C, et al. Onabotulinumtoxin-A in chronic migraine: should timing and definition of non-responder status be revised? Suggestions from a real-life Italian multicenter experience. Headache. 2019;59:1300–9.
Yalinay Dikmen P, Kosak S, Ilgaz Aydinlar E, Sagduyu Kocaman A. A single-center retrospective study of onabotulinumtoxinA for treatment of 245 chronic migraine patients: survey results of a real-world experience. Acta Neurolog Belg. 2018;118:475–84.
Acknowledgements
Funding
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. The journal’s Rapid Service fees were funded by AbbVie.
Editorial Assistance
Abbvie and the authors thank Rachel Danks and Nitesh Singh for assistance with the preparation of this manuscript. Editorial assistance was funded by AbbVie.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Andrew M. Blumenfeld analyzed and interpreted the data, drafted the manuscript, and revised it for intellectual content. Gavneet Kaur, Anadi Mahajan, and Hemlata Shukla acquired, analyzed, and interpreted the data, and drafted the manuscript. Katherine Sommer drafted the manuscript. Amy Tung conceived and designed the study, analyzed and interpreted the data, drafted the manuscript, and revised it for intellectual content. Kerry L. Knievel revised the manuscript for intellectual content. All authors provided final approval of the completed manuscript.
Disclosures
Andrew M. Blumenfeld, MD has served on advisory boards for Allergan, AbbVie, Aeon, Alder, Amgen, Axsome, Biohaven, Impel, Lundbeck, Lilly, Novartis, Revance, Teva, Theranica, and Zoscano; has received funding for speaking from Allergan, AbbVie, Amgen, Biohaven, Lundbeck, Lilly, and Teva; has served as a consultant for Allergan, AbbVie, Alder, Amgen, Biohaven, Lilly, Lundbeck, Novartis, Teva, and Theranica; received grant support from Allergan and Amgen; and has been a contributing author for Allergan, AbbVie, Amgen, Novartis, Teva, Lilly, and Biohaven. Katherine Sommer MRes, PhD and Amy Tung PharmD, MS are employees of AbbVie, and may hold AbbVie stock. Gavneet Kaur, Anadi Mahajan, and Hemlata Shukla are employees of Bridge Medical Consulting, commissioned by AbbVie to carry out this systematic literature review. Kerry Knievel, DO has served as a consultant for AbbVie, Amgen, Lilly, and Biohaven; has conducted research with AbbVie and Amgen; is on speaker programs with AbbVie, Amgen, and Biohaven; and has consulted for Theranica, Upsher-Smith, Lundbeck, Teva, Lilly, and Alder.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Blumenfeld, A.M., Kaur, G., Mahajan, A. et al. Effectiveness and Safety of Chronic Migraine Preventive Treatments: A Systematic Literature Review. Pain Ther 12, 251–274 (2023). https://doi.org/10.1007/s40122-022-00452-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00452-3