Abstract
Background
Immunoglobulins (IG) are widely used for the treatment of a variety of immune-mediated diseases. The exact mechanism of action remains unknown, but IG modulate the expression and function of Fc receptors, interfere with complement activation and production of cytokines, neutralize pathogenic autoantibodies, and affect the activation and effector functions of B and T lymphocytes. Immunoglobulins are usually delivered intravenously, and are effective in ameliorating motor symptoms, and/or preventing disease progression in immune-mediated neuropathies, including Guillain–Barré syndrome and chronic inflammatory demyelinating polyneuropathy.
Objective
The aim of this systematic review and meta-analysis was to study the potential of IG for the treatment of painful peripheral neuropathy (PPN). The outcome of interest was the percentage of patients with PPN who achieved pain relief following IG administration.
Methods
We performed a systematic literature search on March 17, 2022, in the PubMed database without any publication date restrictions. We also looked for unpublished or ongoing trials in clinicaltrials.org. Pain reduction following IG treatment had to be within the aims (primary or secondary).
Results
The aforementioned literature search strategy revealed five studies (two open-label, three randomized placebo-controlled) eligible to be included. The pooled estimate of the percentage of patients with PPN who received immunoglobulins and reported pain relief was found to be 65% (95% CI 58–71%). The likelihood of achieving pain relief with immunoglobulin treatment was 2.9 times higher (95% CI 1.6–5.2) compared to placebo (p = 0.0003).
Conclusion
The use of IG for the treatment of pain due to peripheral neuropathy has a potential therapeutic benefit. Further studies across patients with different types of painful peripheral neuropathy are needed to better characterize this effect.
Registration number on PROSPERO: CRD42022319614.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The pooled percentage of patients with peripheral neuropathy who received immunoglobulins and reported pain relief was found to be 65% (95% CI 58–71%). |
The likelihood of achieving pain relief with immunoglobulin treatment was 2.9 times higher (95% CI 1.6–5.2) compared to placebo (p = 0.0003). |
Immunoglobulins appear to have a potential for the treatment of pain in patients with peripheral neuropathy, however more studies are needed. |
Introduction
Immunoglobulins (IG) are obtained from the plasma pools of healthy blood donors [1, 2]. Five types of human immunoglobulins have been described, IgG, IgM, IgA, IgD, and IgE with the IgG fraction representing nearly 80% of the total amount of immunoglobulin in the human body [1]. IgG consists of a F (ab′)2 fragment that binds to specific antigens and an Fc fragment that exerts effector functions upon binding to Fcγ receptors (FcγRs) [1].
IG are widely used for their immunomodulatory properties for the treatment of a variety of immunedeficiencies and autoimmune diseases [1, 3]. The exact mechanism of action remains unknown, although it is known that IG modulates the expression and function of Fc receptors, interferes with complement activation, preventing complement-mediated cell death and tissue damage [1, 3, 4], neutralizes pathogenic autoantibodies, and affects the activation and effector functions of B and T lymphocytes. Additionally, IG inhibits the activation of monocytes and macrophages, and induces anti-inflammatory cytokines and a direct neutralization effect on innate inflammatory cytokines [5, 6].
For many years, immunoglobulins were preferably administering intravenously, but, in recent years, subcutaneous administration has also been chosen. IG administration may cause adverse events, which most frequently include an injection site reaction, a mildly increased body temperature, and headache. Administration of intravenous immunoglobulin (IVIG) has been shown to have a beneficial effect on ameliorating motor symptoms and/or preventing disease progression of immune-mediated peripheral neuropathies, such as Guillain–Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and multifocal motor neuropathy [7,8,9,10]. IVIG contributes to the treatment of motor symptoms in multiple ways. In particular, through anti-idiotypic effects, it reduces the circulating pathogenic antibody levels (i.e., anti-GM1 IgM) and interferes with antibody-mediated complement deposition in nerves [3]. IVIG also interferes with B-cell receptors on antibody-specific clones, as described in patients with CIDP [11].
Clinical experts using IG in their clinical practice have observed that the latter may have a potential for the treatment of chronic pain in various conditions [12]. These observations have led to the completion and publication of the first clinical trials. The aim of this study was to systematically review the role of IVIG in the treatment of painful peripheral neuropathy (PPN).
Methods
Protocol Registration
This review was initially registered to PROSPERO, an international prospective register database of systematic reviews in health and social care. The registration number was CRD42022319614.
Literature Search Strategy
A systematic literature search was performed on March 17, 2022, in the PubMed database, without any publication date restrictions. For the PubMed search, we used three medical subject heading (MeSH) terms in either the abstract or the title. Term A was “pain” OR “painful”. Term B was “neuropathy” OR “polyneuropathy” OR “neuritis” OR “polyneuritis” OR “mononeuritis” OR “neuronopathy” OR “CIDP” OR “polyradiculoneuropathy” OR “polyradiculopathy”. Term C was “IVIG” OR immunoglobulin” OR “immune globulin”. We also searched at clinicaltrials.gov, a resource provided by the U.S. National Library of Medicine for unpublished trials. For the search, we used the same MeSH terms as above. The filters “with results” AND “completed” were applied. No publication date filter was applied. The reference lists of included articles were further screened to identify further studies that may fall within the scope of this review.
Inclusion Criteria
Articles eligible to be included in this review were required to meet the following criteria:
-
1.
Human subjects were involved.
-
2.
The full article was written in the English language.
-
3.
Pain management was within the primary or secondary aims of the IG use
-
4.
Papers were of adequate methodological quality, as described below.
Exclusion Criteria
Articles meeting the following criteria were excluded from our review:
-
1.
Non-original articles (i.e., review articles, letters, medical hypotheses, etc.).
-
2.
Trials with less than 10 patients per treatment arm.
-
3.
Duplicate articles or papers from the same research teams describing the same patient population.
All article abstracts were screened three times repeatedly in a blinded fashion. Those found to meet any of the exclusion criteria were removed, and any controversies were dealt with consensus during a face-to-face meeting, in which the abstracts were reviewed. All remaining papers were screened again as a full article by at least three reviewers, and conflicts were settled as previously noted.
Quality Assessment of Included Studies
The risk of bias was independently assessed by two of the authors. For the evaluation of randomised controlled trials (RCTs), we used the Cochrane Collaboration’s tool [13, 14], which contains seven evidence-based criteria evaluating selection bias; random sequence generation, allocation concealment, performance bias, detection bias, attrition bias, reporting bias, and other biases [13]. RCTs were considered to be of low risk of bias if low risk of bias was scored in all key domains, unclear risk of bias if low or unclear risk of bias was scored for all key domains, and high risk of bias if high risk of bias was scored for one or more key domains.
The Joanna Briggs Institute Critical Appraisal Checklist was used for the evaluation of case series, which contains ten items [15]. We considered "High risk of bias" studies that met up to 4 of the quality criteria, “Moderate risk of bias” studies that met 5–7 of the quality criteria, and “Low risk of bias” studies that met 8–10 of the quality criteria.
Data Collection Process
After the identification of eligible literature, relevant data were extracted from each study in a structured coding scheme using Excel, and included population size, gender and age distribution, the cause of PPN, the means of diagnosis of the polyneuropathy, the duration of RCT, the response to treatment, the way of assessment of effectiveness, the side effects associated with the treatment, the dropouts associated with the treatment, and the follow-up period of the patients, where applicable. When there was uncertainty considering how data should be interpreted or utilized, at least three authors discussed the study in question to meet consensus.
Data Synthesis
This study used aggregated data where possible, and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [16].
Statistical Analysis
Meta-analysis of the pooled proportions was conducted in the R language [17] using the default settings of the ‘metaprop’ package. The meta-analysis of odds ratios was conducted using the RevMan programme [18], as suggested by the Cochrane Collaboration Group. Heterogeneity between studies was assessed using the I2 statistic [19]. Data were analyzed using a fixed effects model.
Compliance with Ethical Guidelines
This study is based upon studies published prior to the present review. Thus, there are no ethical concerns with respect to this study.
Results
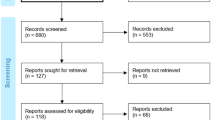
The literature search strategy resulted in the identification of 280 articles. After the eligibility assessment, 175 articles were excluded. In total 5 papers met the inclusion criteria [20,21,22,23,24]. These studies were published between 2015 and 2021. The study selection process is illustrated in the PRISMA Chart (Fig. 1).
Of the included studies, three were placebo-controlled randomized controlled trials and two were retrospective open-label studies. In four studies, IG were administered intravenously, while in one study IG were administered subcutaneously. Two studies included patients with CIDP, one study patients with diabetic peripheral neuropathy (DPN), and two patients with small fiber peripheral neuropathy. The characteristics of the included studies are summarized in Table 1.
The quality assessment of the included papers is available as Supplementary material.
Response to IG Treatment
Figure 2 shows the pooled response to IG administration in patients with PPN who received treatment with IG, following the meta-analysis of the five available studies assessing 265 patients. The pooled response to treatment was 65% (96% CI 58–71%). There was substantial heterogeneity across the included studies (I2 = 90%).
As demonstrated in Fig. 3, the likelihood of responding was 2.9 times higher (95% CI 1.6–5.2) with the administration of IG in comparison to the placebo (p < 0.0003). This was not a statistically significant difference. There was substantial heterogeneity across the included studies (I2 = 62%).
Adverse Events
Common adverse events of IG use included headache, nausea, and dizziness [20, 24]; however, no more dropouts have been reported in the IG-receiving groups compared to placebo [24].
Discussion
In our systematic review and meta-analysis, we looked into the potential of the use of IG administration for the management of PPN. We showed that the use of IG increases the likelihood of ameliorating pain in comparison to placebo by almost three times. This is of particular importance for patients suffering from PPN and for the clinicians treating those patients, as it adds another potential treatment to their armament.
The advantage of our work is that we included papers of adequate methodological quality with well-defined populations of patients suffering from PPN. The diagnoses of peripheral neuropathy had to be based on established and widely accepted criteria. We only included studies where pain management was within the aims of the study after IG administration. Moreover, we searched for unpublished or ongoing trials in order to limit the possibility of not including gray literature.
Although the meta-analysis showed that IG have a potential to treat PPN, our results should be interpreted with caution, given some important limitations. Firstly, the included studies assessed patients with different underlying types of peripheral neuropathy, with the exemption of the studies conducted by Hartung et al. [22] and Kuitwaard et al. [20], who both reported the effectiveness of IG in patients with CIDP. This poses a risk that the underlying pathophysiological mechanisms of the PPN are different. Secondly, the studies we included had different treatment protocols, as well as the researchers used different methods to determine response to treatment (i.e., one point change in Pain Intensity Numerical Rating Scale [24] or 30% reduction of the intensity of pain [21]). Thirdly, the studies followed patients for different periods, and therefore we could not assess the effectiveness of pain at a specific time point after treatment with IG. Finally, a more comprehensive search using other databases rather than PubMed alone may have produced a greater number of articles suitable for final analysis.
Despite these limitations, further well-designed placebo-controlled RTCs are needed to determine the effectiveness of IG in the treatment of PPN. Such studies should focus on immune-mediated neuropathies, given the fact that IG has already a proven effectiveness in treating motor symptoms in such neuropathies. Using widely accepted ways to evaluate pain before and post-treatment at many time points is of utmost importance.
Conclusion
The use of IG for the treatment of PPN has a potential therapeutic benefit. Further studies across patients with PPN of different aetiologies are needed to better characterize this effect.
References
Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491–8.
Patil V, Kaveri SV. The mechanisms of action of IVIG in autoimmune and inflammatory diseases. VOXS. 2013;8:185–8.
Dalakas MC. Mechanisms of action of IVIg and therapeutic considerations in the treatment of acute and chronic demyelinating neuropathies. Neurology. 2002;59(12 Suppl 6):S13-21.
Bouhlal H, Martinvalet D, Teillaud JL, Fridman C, Kazatchkine MD, Bayry J, Lacroix-Desmazes S, Kaveri SV. Natural autoantibodies to Fcγ receptors in intravenous immunoglobulins. J Clin Immunol. 2014;34(Suppl. 1):S4.
Kozicky LK, Zhao ZY, Menzies SC, Fidanza M, Reid GS, Wilhelmsen K, Hellman J, Hotte N, Madsen KL, Sly LM. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol. 2015;98:983.
Ruiz de Souza V, Carreno MP, Kaveri SV, Ledur A, Sadeghi H, Cavaillon JM, Kazatchkine MD, Haeffner-Cavaillon N. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin). Eur J Immunol. 1995;25:1267.
Cats EA, van der Pol WL, Piepers S, Franssen H, Jacobs BC, van den Berg-Vos RM, Kuks JB, van Doorn PA, van Engelen BG, Verschuuren JJ, Wokke JH, Veldink JH, van den Berg LH. Correlates of outcome and response to IVIg in 88 patients with multifocal motor neuropathy. Neurology. 2010;75(9):818–25.
Vucic S, Black KR, Chong PS, Cros D. Multifocal motor neuropathy: decrease in conduction blocks and reinnervation with long-term IVIg. Neurology. 2004;63(7):1264–9.
van der Pol WL, Cats EA, van den Berg LH. Intravenous immunoglobulin treatment in multifocal motor neuropathy. J Clin Immunol. 2010;30(Suppl 1):S79–83.
Gorson KC. An update on the management of chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord. 2012;5(6):359–73.
Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, Lünemann JD. Impaired inhibitory Fc gamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA. 2009;106:4788–92.
Tamburin S, Borg K, Caro XJ, Jann S, Clark AJ, Magrinelli F, Sobue G, Werhagen L, Zanette G, Koike H, Späth PJ, Vincent A, Goebel A. Immunoglobulin g for the treatment of chronic pain: report of an expert workshop. Pain Med. 2014;15(7):1072–82.
Higgins JP, Altman DG, Gøtzsche PC, Jün P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lissy K, Qureshi R, Mattis P, Mu P. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna briggs institute reviewer’s manual. The Joanna Briggs Institute; 2017.
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–33.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559–559.
Review Manager Web (RevMan Web). Version (version number). The Cochrane Collaboration, (version date). Available at revman.cochrane.org (Accessed May 14, 2022).
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Kuitwaard K, Hahn AF, Vermeulen M, Venance SL, van Doorn PA. Intravenous immunoglobulin response in treatment-naïve chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2015;86(12):1331–6.
Liu X, Treister R, Lang M, Oaklander AL. IVIg for apparently autoimmune small-fiber polyneuropathy: first analysis of efficacy and safety. Ther Adv Neurol Disord. 2018;11:1756285617744484.
Hartung HP, Mallick R, Bril V, Lewis RA, Sobue G, Lawo JP, Mielke O, Durn BL, Cornblath DR, Merkies ISJ, van Schaik IN, PATH study group. Patient-reported outcomes with subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy: the PATH study. Eur J Neurol. 2020;27(1):196–203.
Jann S, Fazio R, Cocito D, Toscano A, Schenone A, Marfia GA, Antonini G, De Toni Franceschini L, Mazzeo A, Grandis M, Velardo D, Mataluni G, Peci E. High-dose intravenous immunoglobulin is effective in painful diabetic polyneuropathy resistant to conventional treatments. Results of a double-blind, randomized, placebo-controlled, Multicenter Trial. Pain Med. 2020;21(3):576–85.
Geerts M, de Greef BTA, Sopacua M, van Kuijk SMJ, Hoeijmakers JGJ, Faber CG, Merkies ISJ. Intravenous immunoglobulin therapy in patients with painful idiopathic small fiber neuropathy. Neurology. 2021;96(20):e2534–45.
Acknowledgements
Funding
No funding was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Panagiotis Zis conceived and designed the study, performed the analysis and wrote the paper. Andreas Liampas and Theodora Pozotou collected the data and drafted the paper. Konstantinos Parperis, Artemios Artemiadis and Georgios Hadjigeorgiou critically revised the manuscript. All authors approved of the final version to be published.
Disclosures
Panagiotis Zis, Andreas Liampas, Theodora Pozotou, Konstantinos Parperis, Artemios Artemiadis and Georgios Hadjigeorgiou have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zis, P., Liampas, A., Pozotou, T. et al. Immunoglobulin Use for the Management of Painful Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Pain Ther 11, 1219–1227 (2022). https://doi.org/10.1007/s40122-022-00416-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00416-7