Abstract
Introduction
We compared the effectiveness and virological clearance (VC) at day 7 (T7) post-treatment with molnupiravir, nirmatrelvir/ritonavir, and remdesivir in SARS-CoV-2-infected patients at high risk (HR) for clinical progression.
Methods
We conducted a retrospective study enrolling HR patients with mild-to-moderate COVID-19 (Jan–Oct 2022) treated with nirmatrelvir/ritonavir or molnupiravir or 3 days of remdesivir. We investigated clinical recovery at T7 (resolution of symptoms for ≥ 72 h or all-cause death), VC at T7 (PCR/antigenic negative nasopharyngeal swab), and median time to VC (days from symptom onset to the first negative swab). Factors associated with VC were investigated by logistic regression.
Results
In the study, 92/376 (43.8%) patients received molnupiravir, 150/376 (24.7%) nirmatrelvir/ritonavir, and 134/376 (31.5%) remdesivir. Forty-nine (13%) patients were unvaccinated or incompletely vaccinated. Patients treated with nirmatrelvir/ritonavir were younger and presented immunodeficiencies more frequently; remdesivir was used more commonly in patients hospitalized for other diseases. A high proportion of patients obtained clinical recovery without differences among the therapies (97.5% for molnupiravir, 98.3% for nirmatrelvir/ritonavir, and 93.6% for remdesivir); 12 (3.7%) patients died. Nirmatrelvir/ritonavir was associated with a higher proportion of T7 VC and a shorter time to VC compared to molnupiravir/remdesivir, also after adjustment for age and immunodeficiency (AOR 0.445 RDV vs. NMV-r, 95% CI 0.240–0.826, p = 0.010; AOR 0.222 MNP vs. NMV-r, 95% CI 0.105–0.472, p < 0.001).
Conclusions
SARS-COV-2 antiviral treatments are an excellent therapeutic strategy in HR patients. Nirmatrelvir/ritonavir showed a higher proportion of VC as early as 7 days after treatment, confirming its likely superiority in indirect comparisons.
Plain Language Summary
Nirmatrelvir-ritonavir, molnupiravir, and a 3-day course of remdesivir are antiviral therapies recommended in patients with a mild-to-moderate COVID-19 disease at high risk of clinical progression. Randomized controlled trials and observational studies have shown their efficacy in reducing all-cause mortality and clinical progression. Few data are available about a direct comparison among the three drugs; furthermore, the possible role of nirmatrelvir-ritonavir in increasing viral clearance and in reducing the duration of viral shedding needs to be further elucidated. We thus investigated the effectiveness, safety, and virological clearance 7 days after treatment with these three antivirals in our retrospective cohort. We included in the analysis patients that have received these treatments from January 2022 and October 2022; we observed that patients receiving nirmatrelvir-ritonavir displayed a shorter median time from symptoms’ onset to virological clearance and a higher proportion of virological clearance at day 7, also after adjustment for possible confounders, compared to molnupiravir and remdesivir. Our data might help in understanding which COVID-19 patients may benefit mostly from antiviral therapies and in the choice of antiviral therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We observed a high proportion of clinical recovery in patients with a mild-to-moderate COVID-19 and at high risk of clinical progression following antiviral treatment. |
No differences in clinical recovery were observed among nirmatrelvir/ritonavir, molnupiravir, and a short-course of remdesivir. |
All three of the antiviral therapies are well tolerated with few adverse events. |
Nirmatrelvir/ritonavir was associated with a higher proportion of virological clearance at day 7 after starting the antiviral treatment, compared to molnupiravir and a short course of remdesivir. |
Nirmatrelvir/ritonavir was also associated with a shorter time to virological clearance, compared to molnupiravir and a short course of remdesivir. |
Introduction
The SARS-CoV-2 pandemic has led to the introduction of new drugs and the recovery of old antiviral molecules tested for other infections, such as hepatitis C virus (HCV) or Ebola [1]. So far, the main therapeutic strategies for COVID-19 have included antiviral therapies with different mechanisms of action (remdesivir, molnupiravir, and nirmatrelvir/ritonavir) in outpatients and hospitalized individuals [2].
Antiviral treatments are recommended within 5–7 days from onset of symptoms for patients diagnosed with a mild-to-moderate COVID-19 disease and risk factors for clinical progression to severe disease. The “Agenzia Italiana del Farmaco” (AIFA) defined age > 65 years or chronic comorbidities as well as diabetes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), asthma or other chronic pulmonary diseases, immunodeficiencies, active cancers, neurological diseases and chronic liver or kidney failure as risk factors for severe disease [3].
Among antivirals, nirmatrelvir/ritonavir (NMV/r) is an oral protease inhibitor characterized by a potent pan-human-coronavirus activity in vitro; its target is the viral main protease Mpro [4]. It resulted in 89% relative risk reduction of hospitalization or death in unvaccinated symptomatic high-risk patients before Omicron era, as demonstrated by the EPIC-HR trial [5]. Furthermore, its antiviral activity does not seem to reduce in vitro against the Omicron variant [6]; in fact, observational studies report a reduction in hospitalization and mortality rates also among vaccinated patients during the Omicron era [7,8,9,10,11,12,13,14].
Similar efficacy in reducing clinical progression was observed for remdesivir (RDV), a nucleotide prodrug that is metabolized intracellularly to the active nucleoside triphosphate (ATP), which interferes with viral RNA-dependent polymerase activity [15]. This drug has been approved for emergency use since the first phase of the pandemic in hospitalized patients and afterwards also in outpatients [16,17,18]. The PINETREE trial, in fact, involved non-hospitalized patients who were at high risk for COVID-19 progression and demonstrated that a 3-day course of this intravenous antiviral was associated with a 87% lower risk of progression to severe disease or death than placebo; similarly, data from observational studies and real-life settings confirmed its efficacy [19,20,21].
Finally, a third antiviral, molnupiravir (MNP), an oral, small-molecule prodrug that is metabolized and phosphorylated to active ribonucleoside triphosphate (NHC-TP) and then incorporated into SARS-CoV-2 RNA, causing errors during viral replication with subsequent inhibition [4], was able to reduce the risk of hospitalization or death by 30% in at-risk, unvaccinated adults with COVID-19, as highlighted by the MOVe-OUT trial [22].
Retrospective data during the Omicron wave confirmed that MNP and NMV-r were associated with a significant reduction of all-cause mortality and in-hospital disease progression, while only NMV-r reduced the risk of COVID-19-related hospitalizations [23, 24].
Furthermore, in the EPIC-HR trial, NMV-r was able to reduce viral load in nasopharynx at day 5 compared to placebo [5], but similar virological data in real-life settings and data on direct comparisons of the different antiviral treatments also regarding virological efficacy are still scarce [23, 25,26,27].
We thus compared the effectiveness, safety, and virological clearance 7 days after the start of treatment with NMV-r, MNP, and RDV in SARS-CoV-2-infected patients at high risk of clinical progression during the Omicron era.
Methods
Study Design and Population
We conducted a retrospective study enrolling patients with documented COVID-19 by antigen or reverse transcriptase-polymerase chain reaction (RT-PCR) test on a nasopharyngeal swab who received one antiviral treatment at the COVID-19 antiviral therapy outpatient service or during hospitalization for other reasons than COVID-19 at the San Paolo Hospital in Milan, Italy, from January 2022 to October 2022.
Inclusion criteria were mild-to-moderate disease, no need for supplemental oxygen therapy or hospitalization for COVID-19, one or more comorbidities indicated by the “Agenzia Italiana del Farmaco” (AIFA) as risk factor of developing severe COVID-19 disease, and symptoms onset within 5–7 days. Risk factors for clinical progression included body mass index (BMI) ≥ 30, primary or acquired immunodeficiencies, decompensated diabetes, cardio-cerebrovascular diseases, chronic hepatic or renal failure, chronic pulmonary or neurological diseases, or age ≥ 65 years. Mild-to-moderate disease was defined as signs and symptoms of COVID-19 and oxygen saturation ≥ 94% on room air. Patients hospitalized for COVID-19 were excluded from the analysis; patients were included irrespective of their COVID-19 vaccination status.
Study Procedures
Antiviral treatments that were available in Italy during the study period were (i) oral nirmatrelvir/ritonavir (NMV-r): 300 mg/100 mg twice daily for 5 days (or adjusted dose according to renal glomerular filtrate rate); (ii) molnupiravir (MPN): 800 mg twice daily for 5 days; (iii) intravenous remdesivir (RDV): 200 mg day 1, followed by 100 mg on days 2–3.
Patients were referred to the outpatient clinic by their primary care physician, other specialists or the Emergency Department of San Paolo and San Carlo Hospital or were evaluated after testing positive for SARS CoV-2 infection during hospitalization for other causes. The outpatient clinic was active 7 days a week.
At the first access to the clinic, the patients were visited by an infectious disease specialist and underwent routine blood tests (complete blood count, creatinine, GOT, GPT, lactate dehydrogenase, prothrombin time, C-reactive protein).
On the same day, antiviral treatment was started; the choice of antiviral therapy was made according to AIFA recommendations by the attending physician according to the patients’ clinical characteristics and drug properties (comorbidities, renal failure, drug–drug interactions, and possibility of undergoing intravenous treatment for 3 days). The patients were also evaluated 7 days after starting the antiviral treatment (T7): at T7 patients underwent medical examination, routine blood tests, and a PCR or antigenic nasopharyngeal swab, according to the first method used for diagnosis, to assess virological clearance.
For each patient we collected demographic and clinical data about age, sex, comorbidities, symptoms onset and type of symptoms, day of first SARS-CoV-2-positive test, COVID-19 vaccination status, concomitant therapy with heparin or corticosteroids, outcome at day 7. All data were recorded in an electronic dataset in an anonymous form.
The study has received approval by the Ethic Committee Milano Area 1 (no. 0000677, 01/04/2020) and all the subjects provided informed consent to participate in the study. The study has been conducted according to the World Medical Association and the Declaration of Helsinki.
Statistical Analyses
Categorical data were presented as absolute numbers and percentages, quantitative variables as median, and interquartile range (IQR). Study outcomes were: clinical recovery at day 7 (T7), defined as complete resolution of COVID-19 symptoms for at least 72 h or non-resolution of symptoms or all-cause death; virological clearance at T7, defined by a negative PCR or antigenic swab. We also collected median time to virological clearance (days from symptom onset to the first negative PCR or antigenic nasopharyngeal swab). The occurrence of adverse events was collected at T7.
Comparison among the three treatment groups (NMV-r, MNP, and RDV) were performed by non-parametric Kruskal–Wallis test, Chi-squared test, or Fisher’s exact test, as appropriate. Factors associated with virological clearance at T7 were analyzed by univariable and multivariable logistic regression analyses, adjusting for possible confounders. We also performed the logistic regression analysis on outpatients, excluding patients who received the antiviral treatment during hospitalization. We also compared patients treated within the first 3 days of symptoms onset (very early treated patients) versus patients treated after the third day (early patients), as well as unvaccinated versus vaccinated patients by Mann–Whitney test, Chi-squared test or Fisher’s exact test. Statistical analyses were performed by SPSS software, version 21.0.
Results
Study Population
We enrolled 376 patients in the study period: 150/376 (39.9%) received NMV-r, 92/376 (24.5%) MNP, and 134/376 (35.6%) RDV. Table 1 shows the demographic and clinical characteristics of the study population.
The majority of patients were outpatients (284, 75.5%), while 92 (24.5%) were hospitalized for other reasons than COVID-19. The main reasons for hospitalization were: neurological diseases (16/92, 17.4%), cardiological diseases (14/92, 15.2%), abdominal/urinary diseases (12/92, 13.1%), lung diseases (10/92, 10.9%), cancers (8/92, 8.7%), infectious diseases (7/92, 7.6%), kidney diseases (5/92, 5.4%), orthopedic diseases (4/92, 4.3%), hematological diseases (4/92, 4.3%), and other conditions (12/92, 13.1%).
Median age of study population was 75 years (IQR 63–84) and 167 (44.4%) were females. High-risk criteria were more commonly older age (≥ 65 years, 62.2%), cardiovascular disease (37%) and COPD or chronic pulmonary disease (20.2%). Forty-nine patients were immunocompromised; the main immunodeficiencies were: onco-hematological diseases (22/49, 44.9%), solid organ and bone marrow transplantation (3/49, 6.1%), HIV infection (12/49, 24.5%), and autoimmune diseases (12/49, 24.5%).
About vaccination status, 87% of patients were vaccinated for SARS-CoV-2 with at least one dose; 75.5% had received their first booster, and 57.3% received the last dose of vaccine more than 120 days before SARS-CoV-2 infection.
All outpatients have a mild COVID-19 and did not receive oxygen support; 14/92 (15%) patients who were treated with a short course of RDV during hospitalization received oxygen support: low flows oxygen therapy in 11/14 (78%) patients (nasal cannula or Venturi mask) and high flows oxygen therapy in 3/14 (22%) patients (Reservoir mask).
Comparison of Demographic and Clinical Characteristics Among the Patients Receiving the Three Different Antiviral Therapies
As shown in Table 1, patients treated with NMV-r were younger and more commonly females, compared to patients treated with MNP or RDV. Furthermore, patients receiving NMV-r presented more frequently age < 65 years and immunodeficiency as risk factor for severity of COVID-19; patients suffering from cardiovascular diseases, diabetes, and chronic kidney failure received mainly MNP or RDV. Short-course RDV was more frequently used in older patients and in those hospitalized for conditions other than COVID-19.
Outcome and Safety at Day 7 Post Treatment According to Antiviral Treatment
Of 322 patients for whom post-treatment follow-up was available, 309 (96%) patients completely recovered at T7. Twelve (3.7%) patients died (two patients receiving NMV-r, two patients receiving MNP, and eight patients receiving RDV); five of 12 (41.7%) patients died from malignancy, five (41.7%) for sepsis, one (8.3%) for acute cardiovascular failure, and one (8.3%) for cerebral hemorrhage. No difference in T7 outcome was observed among the three different treatments (recovery was 98.3% in NMV-r group, 97.5% in MNP group and 93.6% in RDV group) (Table 1).
All the three therapies were well tolerated with few and mild adverse events (11/322, 2.9%). NMV-r was characterized by a higher proportion of adverse events (9, 6%), mostly dysgeusia; no serious event was displayed (Table 1). Blood tests showed higher median levels of CRP at start of treatment in patients treated with RDV (RDV: 10.9, IQR 5.4–42.3 mg/l; NMV-r: 4.9, IQR 4.9–8.3; MNP: 5.9, IQR 4.9–9.4; p < 0.001); no increase in GOT/GPT or creatinine was observed for any treatments between start of treatment and T7.
Virological Clearance According to Antiviral Treatment
There were 250 of 376 patients that underwent nasopharyngeal swab at day 7 (T7) after starting therapy. Virological clearance at T7 was obtained in nearly one-third of patients (111/250, 29.5%).
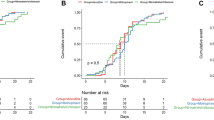
Interestingly, a higher percentage of patients reached virological clearance at T7 in the NMV-r group (53/85, 62.4%) in comparison with MNP (15/59, 25.4%) and RDV (43/109, 39.4%; p < 0.001). Patients treated with NMV-r also showed a shorter median time from symptom onset to the first negative nasopharyngeal swab compared to the other treatments (10 days, IQR 8–15 for NMV-r; 14, IQR 11–18 for MNP; 13, IQR 10–19 for RDV; p = 0.002) (Table 1).
The association between NMV-r and higher proportion of virological clearance at T7 was also investigated by fitting a multivariable logistic regression analysis, adjusting for possible confounders (age and immunodeficiency, which are well-known risk factors for viral persistence). Table 2 shows the results of univariable and multivariable logistic regression analyses. After correction for age and immunodeficiency, treatment with NMV-r was confirmed to be associated with a higher proportion of virological clearance (AOR 0.445 RDV versus NMV-r, 95% CI 0.240–0.826, p = 0.010; AOR 0.22 MNP versus NMV-r, 95% CI 0.105–0.472, p < 0.001). The higher probability of viral clearance following treatment with NMV-r compared to RDV and MNP was also confirmed in the analyses restricted to outpatients (Supplementary Table 1).
Efficacy and Safety of Antiviral Treatment According to Timing of Treatment and Vaccination Status
Two hundred and fifty-seven of 346 (74%) patients received very early antiviral treatment (within the first 3 days of symptoms onset); no difference in efficacy and safety of the three antiviral treatments was shown according to the time of treatment (very early treatment versus early treatment, i.e., at least 3 days after symptoms onset) (Table 3). Median time to virological clearance was shorter in very early treated patients, while no difference in the proportion of virological clearance at T7 was observed.
Twenty-nine of 356 (8.2%) patients were unvaccinated against COVID-19 (Table 4). Interestingly, while displaying similar clinical recovery and adverse events, unvaccinated patients showed a longer time to virological clearance.
Discussion
Randomized controlled trials and observational studies have shown the efficacy and safety of antiviral treatments for SARS-CoV-2 infection also during the Omicron wave and in vaccinated patients: NMV-r, MNP, and RDV are associated with a reduced risk of clinical progression and death, especially in older patients or patients at high risk for clinical progression [5, 8, 9, 11,12,13, 19, 22,23,24, 28, 29].
In our retrospective study, we observed that antiviral treatments succeeded in obtaining a clinical cure in more than 90% of patients without differences among the three drugs; RDV has a slight non-significant minor proportion of clinical recovery, but appears also more commonly prescribed in older hospitalized patients. Few deaths were recorded and about half of patients died from complications not directly related to COVID-19, but to the underlying oncological disease.
These data confirmed results from randomized trials that were all conducted in a period before Omicron variant and in unvaccinated patients [5, 19, 22]; afterwards, observational studies including emulation trials have reported effectiveness of NMV-r, MNP, or RDV, also in vaccinated patients, in Omicron era and compared to patients that have not received any antiviral treatment [7, 8, 23, 24, 29, 30]. In fact, all three treatments retain effectiveness against the Omicron variant, as demonstrated by Vangeel et al. and other authors [6, 31]. The effectiveness of these treatments is evident despite the reduced mortality due to COVID-19 observed with the currently circulating viral variants and is in any case associated with cost savings thanks to avoided hospitalizations [32].
Among these treatments, MNP seems characterized by a lower reduction in the risk of hospitalization and mortality: in the MOVe-OUT trial its relative risk reduction was only 30% compared to placebo; likewise, no effect was observed in reducing COVID-19-associated hospital admissions or death versus standard of care in PANORAMIC trial [33]. Furthermore, other trials and observational studies displayed a reduction in all-cause mortality, but not all found an effect of MNP in reduction of disease progression [29, 34, 35]. However, we observed a high proportion of clinical recovery also in MNP-treated patients, similarly to the other two therapies, in line with another Italian retrospective analysis [36].
As regards safety issues, all the three drugs were well tolerated with a very low prevalence of adverse events, confirming data from trials and real-life data [5, 7, 22, 23]. A higher proportion of adverse events was reported with NMV-r compared to MNP and RDV, but no severe adverse events and no discontinuation were observed. The prevalence of side effects was comparable to that reported by other retrospective studies and NMV-r was not associated with a higher proportion of adverse events compared to placebo in both trials and meta-analyses [4, 37, 38].
Interestingly, in our study NMV-r seemed to have a better and faster virological response: virological clearance 1 week after the start of treatment was reached in more than half of patients receiving NMV-r, but only in about a third of patients treated with MNP or RDV. NMV-r also showed a shorter time to the first negative PCR or antigenic nasopharyngeal swab. The higher proportion of viral response in a short period for NMV-r, compared to RDV and MNP, was independent from age and immunosuppression conditions.
A faster trend of conversion from positive to negative RT-PCR RNA with NMV-r versus placebo was expected and already known [26, 39]; furthermore, the higher probability of having a negative SARS-CoV-2 swab within 10 days from the first positive one following treatment with NMV-r was previously described, also after controlling for possible confounders, but in comparison only with RDV and not MNP [36]. Nevertheless, a recent Cochrane systematic review failed to find a certain effect of NMV-r in increasing viral clearance at 7 and 14 days and other authors found that NMV-r effectively reduced viral loads, but was not able to decrease the length of virus shedding [27, 40].
Obtaining a faster virological response is crucial to shorten time of isolation and contagiousness, mainly in patients who need diagnostic procedures or the continuation of life-saving therapies.
A shorter time to viral clearance was reported also for patients treated very early after symptoms onset and for vaccinated patients, suggesting the importance of a timely initiation of antiviral treatment and the completion of vaccination schedule especially in patients who would be disadvantaged by long periods of isolation due to concomitant pathologies.
Our study has some limitations: the retrospective nature and the lack of a control group of patients not receiving any antiviral treatment; unmeasured confounders for the association between antiviral treatment and virological clearance; the lack of daily nasopharyngeal swabs to assess the actual time to viral response; the limited sample size and losses to follow-up. This is, however, one of the first studies directly comparing three different antiviral treatments for high-risk outpatients in a real-life setting during the Omicron era.
Conclusions
In conclusion, NMV-r, MNP, and RDV have been confirmed as a therapeutic strategy for high-risk patients diagnosed with mild COVID-19 disease in order to reduce disease progression without differences among the three drugs; NMV-r was associated with a higher, but still overall low proportion, of adverse events. A possible better performance of this antiviral, compared to the other therapies, is its ability to shorten the period of viral shedding and disease transmission, which is even now essential for some categories of patients such as subjects diagnosed with hematological malignancies.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J. Remdesivir against COVID-19 and Other Viral Diseases. Clin Microbiol Rev. 2020. https://doi.org/10.1128/CMR.00162-20.
Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, Lytvyn L, Leo YS, Macdonald H, Zeng L, Amin W, da Silva ARA, Aryal D, Barragan FAJ, Bausch FJ, Burhan E, Calfee CS, Cecconi M, Chacko B, Chanda D, Dat VQ, De Sutter A, Du B, Freedman S, Geduld H, Gee P, Gotte M, Harley N, Hashimi M, Hunt B, Jehan F, Kabra SK, Kanda S, Kim YJ, Kissoon N, Krishna S, Kuppalli K, Kwizera A, Lado Castro-Rial M, Lisboa T, Lodha R, Mahaka I, Manai H, Mendelson M, Migliori GB, Mino G, Nsutebu E, Preller J, Pshenichnaya N, Qadir N, Relan P, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, Swanstrom R, Ugarte S, Uyeki T, Venkatapuram S, Vuyiseka D, Wijewickrama A, Tran L, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Kawano-Dourado L, Jacobs M, Vandvik PO. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370: m3379. https://doi.org/10.1136/bmj.m3379.
AIFA, Agenzia Italiana del Farmaco Elenchi farmaci di classe A e H. 2020–15–07; https://www.aifa.gov.it/liste-farmaci-a-h2020
Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis. 2023;76(1):165–71. https://doi.org/10.1093/cid/ciac180.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM, Investigators E-H. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397–408. https://doi.org/10.1056/NEJMoa2118542.
Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, Leyssen P, Neyts J, Jochmans D. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198: 105252. https://doi.org/10.1016/j.antiviral.2022.105252.
Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, Friger M, Waxman JG, Dagan N, Balicer R, Ben-Shlomo Y, Peretz A, Yaron S, Serby D, Hammerman A, Netzer D. Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–8. https://doi.org/10.1056/NEJMoa2204919.
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, Goldstein LH, Saliba W. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342–9. https://doi.org/10.1093/cid/ciac443.
Kaboré JL, Laffont B, Diop M, Tardif MR, Turgeon AF, Dumaresq J, Luong ML, Cauchon M, Chapdelaine H, Claveau D, Brosseau M, Haddad E, Benigeri M. Real-world effectiveness of nirmatrelvir/ritonavir on coronavirus disease 2019-associated hospitalization prevention: a population-based cohort study in the Province of Quebec, Canada. Clin Infect Dis. 2023;77(6):805–15. https://doi.org/10.1093/cid/ciad287.
Al-Obaidi MM, Gungor AB, Murugapandian S, Thajudeen B, Mansour I, Wong RC, Tanriover B, Zangeneh TT. The impact of nirmatrelvir-ritonavir in reducing hospitalizations among high-risk patients with SARS-CoV-2 during the omicron predominant era. Am J Med. 2023;136(6):577–84. https://doi.org/10.1016/j.amjmed.2023.02.022.
Mutoh Y, Umemura T, Nishikawa T, Kondo K, Nishina Y, Soejima K, Noguchi Y, Bando T, Ota S, Shimahara T, Hirota S, Hagimoto S, Takei R, Fukihara J, Sasano H, Yamano Y, Yokoyama T, Kataoka K, Matsuda T, Kimura T, Ichihara T, Kondoh Y. Real-world experience of the comparative effectiveness and safety of molnupiravir and nirmatrelvir/ritonavir in high-risk patients with COVID-19 in a community setting. Viruses. 2023. https://doi.org/10.3390/v15030811.
Aggarwal NR, Molina KC, Beaty LE, Bennett TD, Carlson NE, Mayer DA, Peers JL, Russell S, Wynia MK, Ginde AA. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. Lancet Infect Dis. 2023;23(6):696–705. https://doi.org/10.1016/S1473-3099(23)00011-7.
Lewnard JA, McLaughlin JM, Malden D, Hong V, Puzniak L, Ackerson BK, Lewin BJ, Kim JS, Shaw SF, Takhar H, Jodar L, Tartof SY. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023;23(7):806–15. https://doi.org/10.1016/S1473-3099(23)00118-4.
Schwartz KL, Wang J, Tadrous M, Langford BJ, Daneman N, Leung V, Gomes T, Friedman L, Daley P, Brown KA. Population-based evaluation of the effectiveness of nirmatrelvir-ritonavir for reducing hospital admissions and mortality from COVID-19. CMAJ. 2023;195(6):E220–6. https://doi.org/10.1503/cmaj.221608.
Kokic G, Hillen HS, Tegunov D, Dienemann C, Seitz F, Schmitzova J, Farnung L, Siewert A, Höbartner C, Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat Commun. 2021;12(1):279. https://doi.org/10.1038/s41467-020-20542-0.
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM, Investigators G-U--. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57. https://doi.org/10.1001/jama.2020.16349.
Consortium WST. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941–53. https://doi.org/10.1016/S0140-6736(22)00519-0.
Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, Wang S, Walunas TL, Swaminathan S, Slim J, Chin B, De Wit S, Ali SM, Soriano Viladomiu A, Robinson P, Gottlieb RL, Tsang TYO, Lee IH, Hu H, Haubrich RH, Chokkalingam AP, Lin L, Zhong L, Bekele BN, Mera-Giler R, Phulpin C, Edgar H, Gallant J, Diaz-Cuervo H, Smith LE, Osinusi AO, Brainard DM, Bernardino JI, Investigators G-U-aG-U-. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) versus a cohort receiving standard of care. Clin Infect Dis. 2021;73(11):e4166–74. https://doi.org/10.1093/cid/ciaa1041.
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA, Investigators G-U-P. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386(4):305–15. https://doi.org/10.1056/NEJMoa2116846.
Ramos-Rincón JM, Pinargote-Celorio H, Llenas-García J, Moreno-Pérez O, González-Cuello I, Gonzalez-de-la-Aleja P, Martínez-López B, Reus S, García-López M, Rodríguez JC, Boix V, Merino E. A retrospective real-world study of early short-course remdesivir in non-hospitalized COVID-19 patients at high risk for progression: low rate of hospitalization or death, regardless of immunocompetence status. Front Pharmacol. 2023;14:1218650. https://doi.org/10.3389/fphar.2023.1218650.
Solera JT, Árbol BG, Bahinskaya I, Marks N, Humar A, Kumar D. Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave. Am J Transplant. 2023;23(1):78–83. https://doi.org/10.1111/ajt.17199.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, Du J, Pedley A, Assaid C, Strizki J, Grobler JA, Shamsuddin HH, Tipping R, Wan H, Paschke A, Butterton JR, Johnson MG, De Anda C, Group M-OS. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20. https://doi.org/10.1056/NEJMoa2116044.
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–93. https://doi.org/10.1016/S1473-3099(22)00507-2.
Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Mutalik P, Yan L, Cunningham F, Hynes DM, Rowneki M, Bohnert A, Boyko EJ, Iwashyna TJ, Maciejewski ML, Osborne TF, Viglianti EM, Aslan M, Huang GD, Ioannou GN. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med. 2023;176(6):807–816. www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-3565.
Amani B, Akbarzadeh A, Shabestan R, Khorramnia S, Navidi Z, Rajabkhah K, Kardanmoghadam V. Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis. J Med Virol. 2023;95(6): e28889. https://doi.org/10.1002/jmv.28889.
Cegolon L, Pol R, Simonetti O, Larese Filon F, Luzzati R. Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life. Pharmaceuticals (Basel). 2023. https://doi.org/10.3390/ph16050721.
Kim H, Yang JS, Ko JH, Lee M, Lee JY, Park S, Kim JW, Shin Y, Lee JM, Na YJ, Park BK, Lee YH, Yang J, Huh K, Cho SY, Kang CI, Chung DR, Peck KR. Can nirmatrelvir/ritonavir treatment shorten the duration of COVID-19 isolation? Front Med (Lausanne). 2022;9: 988559. https://doi.org/10.3389/fmed.2022.988559.
Dryden-Peterson S, Kim A, Kim AY, Caniglia EC, Lennes IT, Patel R, Gainer L, Dutton L, Donahue E, Gandhi RT, Baden LR, Woolley AE. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a population-based cohort study. Ann Intern Med. 2023;176(1):77–84. www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-2141. https://doi.org/10.7326/M22-2141
Wan EYF, Yan VKC, Wong ZCT, Chui CSL, Lai FTT, Li X, Wong CKH, Hung IFN, Lau CS, Wong ICK, Chan EWY. Effectiveness of molnupiravir vs. nirmatrelvir-ritonavir in non-hospitalised and hospitalised patients with COVID-19: a target trial emulation study. EClinicalMedicine. 2023;64: 102225. https://doi.org/10.1016/j.eclinm.2023.102225.
Piccicacco N, Zeitler K, Ing A, Montero J, Faughn J, Silbert S, Kim K. Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge. J Antimicrob Chemother. 2022;77(10):2693–700. https://doi.org/10.1093/jac/dkac256.
Li P, Wang Y, Lavrijsen M, Lamers MM, de Vries AC, Rottier RJ, Bruno MJ, Peppelenbosch MP, Haagmans BL, Pan Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32(3):322–4. https://doi.org/10.1038/s41422-022-00618-w.
Wai AK, Chan CY, Cheung AW, Wang K, Chan SC, Lee TT, Luk LY, Yip ET, Ho JW, Tsui OW, Cheung KW, Lee S, Tong CK, Yamamoto T, Rainer TH, Wong EL. Association of molnupiravir and nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2023;30: 100602. https://doi.org/10.1016/j.lanwpc.2022.100602.
Butler CC, Hobbs FDR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, Dorward J, Lowe DM, Standing JF, Breuer J, Khoo S, Petrou S, Hood K, Nguyen-Van-Tam JS, Patel MG, Saville BR, Marion J, Ogburn E, Allen J, Rutter H, Francis N, Thomas NPB, Evans P, Dobson M, Madden TA, Holmes J, Harris V, Png ME, Lown M, van Hecke O, Detry MA, Saunders CT, Fitzgerald M, Berry NS, Mwandigha L, Galal U, Mort S, Jani BD, Hart ND, Ahmed H, Butler D, McKenna M, Chalk J, Lavallee L, Hadley E, Cureton L, Benysek M, Andersson M, Coates M, Barrett S, Bateman C, Davies JC, Raymundo-Wood I, Ustianowski A, Carson-Stevens A, Yu LM, Little P, Group PTC. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–93. https://doi.org/10.1016/S0140-6736(22)02597-1.
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, Goldstein LH, Saliba W. Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis. Clin Infect Dis. 2023;76(3):453–60. https://doi.org/10.1093/cid/ciac781.
Khoo SH, FitzGerald R, Saunders G, Middleton C, Ahmad S, Edwards CJ, Hadjiyiannakis D, Walker L, Lyon R, Shaw V, Mozgunov P, Periselneris J, Woods C, Bullock K, Hale C, Reynolds H, Downs N, Ewings S, Buadi A, Cameron D, Edwards T, Knox E, Donovan-Banfield I, Greenhalf W, Chiong J, Lavelle-Langham L, Jacobs M, Northey J, Painter W, Holman W, Lalloo DG, Tetlow M, Hiscox JA, Jaki T, Fletcher T, Griffiths G, Group AC-S. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis. 2023;23(2):183–95. https://doi.org/10.1016/S1473-3099(22)00644-2.
Tiseo G, Barbieri C, Galfo V, Occhineri S, Matucci T, Almerigogna F, Kalo J, Sponga P, Cesaretti M, Marchetti G, Forniti A, Caroselli C, Ferranti S, Pogliaghi M, Polidori M, Fabiani S, Verdenelli S, Tagliaferri E, Riccardi N, Suardi LR, Carmignani C, Batini S, Puccetti L, Iapoce R, Menichetti F, Falcone M. Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience. Infect Dis Ther. 2023;12(1):257–71. https://doi.org/10.1007/s40121-022-00729-2.
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, Wang M, Mao Q. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516–23. https://doi.org/10.1080/07853890.2022.2034936.
Park JJ, Lee J, Seo YB, Na SH. Nirmatrelvir/ritonavir prescription rate and outcomes in coronavirus disease 2019: a single center study. Infect Chemother. 2022;54(4):757–64. https://doi.org/10.3947/ic.2022.0123.
Li H, Gao M, You H, Zhang P, Pan Y, Li N, Qin L, Wang H, Li D, Li Y, Qiao H, Gu L, Xu S, Guo W, Wang N, Liu C, Gao P, Niu J, Cao J, Zheng Y. Association of nirmatrelvir/ritonavir Treatment on upper respiratory severe acute respiratory syndrome coronavirus 2 reverse transcription-polymerase chain reaction (SARS-Cov-2 RT-PCR) negative conversion rates among high-risk patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2023;76(3):e148–54. https://doi.org/10.1093/cid/ciac600.
Reis S, Metzendorf MI, Kuehn R, Popp M, Gagyor I, Kranke P, Meybohm P, Skoetz N, Weibel S. Nirmatrelvir combined with ritonavir for preventing and treating COVID-19. Cochrane Database Syst Rev. 2022;9(9): e015395. https://doi.org/10.1002/14651858.CD015395.pub2.
Acknowledgements
We are thankful to all the patients who participated in the study and their families. We would like to thank all the staff of the Clinic of Infectious Diseases and Tropical Medicine, San Paolo Hospital, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan who cared for the patients.
Funding
EuCARE project, funded by the European Union’s Horizon Europe Research and Innovation Programme under Grant Agreement No 101046016. The journal’s Rapid Service fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Giulia Marchetti, Antonella d’Arminio Monforte, and Francesca Bai developed the question research and the study protocol. Francesca Bai, Tomaso Beringheli, Virginia Vitaletti, Andrea Santoro, Francesco Molà, Alessandro Copes, Nicole Gemignani, Sofia Pettenuzzo, Roberto Castoldi, Benedetta Varisco, Riccardo Nardo, Lorenzo Brando Lundgren, Lorenzo Albertini, Riccardo Ligresti, Matteo Sala, Matteo Augello, Lorenzo Biasioli, and Teresa Bini helped with patients’ recruitment; Valeria Bono and Roberta Rovito helped with collection of data. Francesca Bai performed statistical analyses. Sabrina Passarella and Nicola Vincenzo Orfeo were in charge of the management of the COVID-19 inpatient and outpatient services organization. Francesca Bai and Tomaso Beringheli analyzed and interpreted the data and wrote the manuscript. Giulia Marchetti and Antonella d’Arminio Monforte helped in analyzing and interpreting the data. Giulia Marchetti and Antonella d’Arminio Monforte contributed to the final data interpretation. All authors contributed to the editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Francesca Bai, Tomaso Beringheli, Virginia Vitaletti, Andrea Santoro, Francesco Molà, Alessandro Copes, Nicole Gemignani, Sofia Pettenuzzo, Roberto Castoldi, Benedetta Varisco, Riccardo Ligresti, Matteo Sala, Lorenzo Albertini, Matteo Augello, Lorenzo Biasioli, Valeria Bono, Roberta Rovito, Teresa Bini, Sabrina Passarella, Nicola Vincenzo Orfeo, Antonella d’Arminio Monforte, and Giulia Marchetti declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Ethical Approval
The study has received approval by the Ethic Committee Milano Area 1 (n. 0000677, 01/04/2020) and all the subjects provided informed consent to participate in the study. The study has been conducted according to the World Medical Association and the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: Preliminary results were presented at the HIV Drug Therapy Glasgow Conference, 23rd–26th October, 2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bai, F., Beringheli, T., Vitaletti, V. et al. Clinical Outcome and 7-Day Virological Clearance in High-Risk Patients with Mild–Moderate COVID-19 Treated with Molnupiravir, Nirmatrelvir/Ritonavir, or Remdesivir. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00994-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-00994-3