Abstract
Introduction
Different antivirals are available for the treatment of outpatients with COVID-19. Our aim was to describe a real-world experience of outpatient management of COVID-19 subjects at high risk of progression.
Methods
This prospective observational study conducted in the University Hospital of Pisa (January 2022–July 2022) included consecutive COVID-19 outpatients with at least one risk factor for disease progression. Patients received nirmatrelvir/ritonavir, molnupiravir, or 3-day remdesivir, according to the Italian Medicines Agency (AIFA) indications. All patients were followed up until 30 days from the first positive nasopharyngeal swab. The primary endpoint was a composite of death or hospitalization. Secondary endpoints were occurrence of adverse events and a negative test within 10 days from the first positive test. Multivariable analysis was performed to identify factors associated with death or hospitalization.
Results
Overall, 562 outpatients were included: 114 (20.3%) received molnupiravir, 252 (44.8%) nirmatrelvir/ritonavir, and 196 (34.9%) 3-day remdesivir. The composite endpoint occurred in 2.5% of patients and was more frequent in patients treated with remdesivir (5.1%) compared with molnupiravir (1.8%) or nirmatrelvir/ritonavir (0.8%, ANOVA among groups p = 0.012). On multivariable Cox regression analysis, presence of ≥ 3 comorbidities, hematological disease, gastrointestinal symptoms, and each-day increment from symptoms onset were factors associated with death or hospitalization, while antiviral treatment was not a predictor. Adverse events occurred more frequently in the nirmatrelvir/ritonavir group (49.2%). Nirmatrelvir/ritonavir compared with remdesivir was associated with a higher probability of having a negative test within 10 days from the first positive one.
Conclusion
Death or hospitalization did not differ among high-risk COVID-19 outpatients treated with currently available antivirals. Safety and time to a negative test differed among the three drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this real-world study, the risk of COVID-19 progression was not statistically different in high-risk patients treated with nirmatrelvir/ritonavir, molnupiravir, or 3-day remdesivir. |
Death or hospitalization in COVID-19 outpatients treated with available antivirals was 2.5%. |
Adverse events were more frequent in patients who received nirmatrelvir/ritonavir. |
Nirmatrelvir/ritonavir compared with remdesivir was associated with a higher likelihood of having a negative test within 10 days of the first positive one. |
Introduction
Since the start of the pandemic, a variety of prophylactic and therapeutic treatments have been developed to combat COVID-19 [1, 2]. Monoclonal antibodies (mAbs) initially demonstrated a reduction in hospitalizations or deaths by 70–87% among outpatients, and represented an effective therapeutic option in vulnerable populations before or after exposure to SARS-CoV-2 [3]. However, the neutralizing ability of mAbs progressively decreased with the emergence of new variants of concern (VOC) [4]. The recent emergence of the heavily mutated Omicron variant has posed a challenge to this treatment strategy. As a matter of fact, all available mAbs, including sotrovimab and tixagevimab, seem to have no or reduced neutralizing activity against the last Omicron BA.2 and BA.5 variants [5, 6]. From December 2021, new therapeutic options for the outpatient management of COVID-19 have been authorized by regulatory agencies [7,8,9]. Nirmatrelvir/ritonavir, molnupiravir, and early 3-day remdesivir have been approved as outpatient treatments for patients at increased risk of progression. Interestingly, the susceptibilities of Omicron BA.2 to remdesivir, molnupiravir, and nirmatrelvir are similar to those of the ancestral strain and other VOCs [10]. Phase 3 clinical studies showed different efficacy of the three agents. Molnupiravir demonstrated a reduced risk of hospitalization or death compared with placebo [7.3% versus 14.1%, −6.8 percentage points difference; 95% confidence interval (CI) −11.3 to −2.4, p = 0.001] [7]. In some reports, molnupiravir has been found to reduce the risk of hospitalization or death by approximately 50% [11]. The randomized controlled trial on nirmatrelvir/ritonavir, reported that 3 out of 389 patients (0.77%) in the nirmatrelvir group versus 27/385 (7%) in the placebo group (−6.32 percentage points difference, 95% CI 9.04 to −3.59, p < 0.001) had hospitalization or death by day 28 [8]. The relative risk reduction was 89.1% [8]. Similarly, in the randomized controlled trial by Gottlieb and colleagues, 2 out of 279 patients (0.7%) in the remdesivir group versus 15 out of 283 (5.3%) in the placebo group had a COVID-19–related hospitalization by day 28, with a relative risk reduction of 87% in the remdesivir group [9].
The aim of this study is to describe a real-world experience of outpatient management of patients with high risk of progression to severe COVID-19 who received nirmatrelvir/ritonavir, molnupiravir, or 3-day remdesivir.
Methods
Study Design
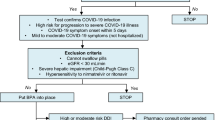
This is a prospective observational study conducted in the University Hospital of Pisa (Italy) from 1 January 2022 to 1 July 2022. Consecutive outpatients with documented COVID-19 by antigen or reverse transcriptase–polymerase chain reaction (RT–PCR) test on a nasopharyngeal swab who received one authorized antiviral treatment were eligible for the study. Eligible patients were referred to our ambulatory service by the family physician or other outpatient services through a dedicated phone system active 12 h daily 7 days a week. Patients were eligible for the treatment if they did not require supplemental oxygen therapy, were not hospitalized due to COVID-19, and had mild-to-moderate COVID-19. Mild and moderate illness were defined according to National Institute of Health (NIH) guidelines [mild disease: individuals who have any of the various signs and symptoms of COVID-19 but who do not have shortness of breath, dyspnea, or abnormal chest imaging; moderate disease: individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation measured by pulse oximetry (SpO2) ≥ 94% on room air] [12]. Patients were eligible if they had mild-to-moderate COVID-19 and at least one of the risk factors associated with progression to severe disease reported in Table 1. Asymptomatic patients were not included according to indications from the Italian Medicines Agency (AIFA).
Included patients received an ambulatory visit, which included collection of medical history, physical examination, vital signs evaluation, and blood examinations. Information about comorbidities, symptoms onset, and medications were collected by the attending physician. Patients were then allocated to one of the three available treatments according to inclusion/exclusion criteria for each antiviral (see below) and presence of potential drug–drug interactions. The final decision to start one of the three antivirals was made according to AIFA recommendations and based on the judgment of the attending physician. The ambulatory was active 7 days a week.
All patients were followed-up until 30 days from their first positive nasopharyngeal swab. Follow-up included a new ambulatory visit or, alternatively, a phone visit at days 7 and 30 from the start of antiviral treatment. Patients repeated antigenic nasopharyngeal swabs at day 7 and then, if still positive, on days 10 and 14 from the first positive nasopharyngeal swabs or until a negative test. The occurrence of adverse events (AEs) was recorded through the administration of a dedicated questionnaire by members of the study team at the end of the study.
Compliance with Ethics Guidelines
All patients signed a written informed consent. The study was conducted according to the principles stated in the Declaration of Helsinki, and approved by the local Ethical Committee of the Area Vasta Nord Ovest of Tuscany region (IRB number 230320).
Treatment Exposure
Patients received one of the three authorized antivirals according to indications from AIFA. There are some differences in the timing, administration, and contraindications for the three antivirals (Table 1).
Nirmatrelvir/ritonavir consists of nirmatrelvir, a novel SARS-CoV-2 main protease inhibitor targeting 3CLpro of SARS-CoV-2, plus ritonavir, an inhibitor of cytochrome P-450 3A4 to decrease nirmatrelvir metabolism that increases its serum levels [13]. It was prescribed within 5 days from the onset of symptoms and administered for a full 5-day treatment oral course. Dosage was adjusted according to renal function: in patients with estimated glomerular filtration rate (GFR) > 60 ml/min nirmatrelvir/ritonavir was administered at the dose of 300 mg of nirmatrelvir plus 100 mg of ritonavir twice daily, in patients with GFR 30–60 ml/min at a dose of 150 mg of nirmatrelvir plus 100 mg of ritonavir, and in patients with GFR < 30 ml/min it was not used.
Molnupiravir, an inhibitor of the RNA-dependent RNA polymerase (RdRp) enzyme of SARS-CoV-2 [14], was administered within 5 days from symptoms onset at the dose of 800 mg twice for a full 5-day oral course. No dose adjustment was made according to renal function.
Remdesivir was administered within 7 days from symptoms onset at the dose of 200 mg intravenously (iv) the first day and 100 mg iv the second and third day (3-day course). No dose adjustment was made according to renal function, but was not administered in patients with GFR < 30 ml/min.
Study Outcomes
The primary composite endpoint is death or hospitalization for COVID-19 in the overall population and among the three treatment groups.
The secondary study endpoints are: occurrence of AEs and negative nasopharyngeal swab within 10 days from the first positive test.
Study Variables and Definitions
For each patient, sociodemographic data including age and sex were collected. Data on comorbidities and conditions associated with high risk for severe COVID-19, including body mass index (BMI), diabetes mellitus, hypertension, cardiovascular and cerebrovascular disease, chronic lung disease, chronic liver disease, chronic kidney disease, neurological disorders, immunosuppression, solid cancer, hematological disease, autoimmune disease, solid organ transplantation, and neurological disease were recorded. Immunosuppression was defined by the presence of acquired immunosuppressive state or use of immunosuppressive therapy such as steroids (prednisolone > 0.5 mg/kg/d or equivalent for > 1 month), chemotherapy, or antitumor necrosis factor therapy, or the use of tacrolimus, cyclosporin, and/or mycophenolate. Obesity was defined by a BMI > 30 kg/m2.
In addition, information about COVID-19 vaccination dates were recorded. COVID-19 vaccination status was classified into two categories: adequate versus nonadequate, based on the timing of the last vaccine dose before study entry, as previously described [15]. Unvaccinated subjects and those who received only the first vaccine dose were considered nonadequately vaccinated. For the second vaccine dose and subsequent doses given more than 180 days apart, a patient was considered to be adequately vaccinated if he/she received the last dose in the previous 8–180 days. If the gap between the last two doses was less than 180 days, a patient was considered adequately vaccinated starting from the date of the last vaccine dose up to 180 days after [15].
Information about symptoms related to COVID-19 was collected. The attending physician investigated the presence of fever, fatigue, cough, dyspnea, myalgias, and gastrointestinal symptoms (including nausea, vomiting, and diarrhea) in each patient.
Regarding the occurrence of an AE, AEs indicated any untoward medical occurrence associated with the use of the antiviral, whether or not they were considered drug related. Data about the occurrence of AEs requiring drug discontinuation were collected. Data about hospitalization due to COVID-19 or death were recorded. Hospitalized patients were treated as previously described [16].
Statistical Analysis
Continuous variables were summarized with mean and standard deviation (SD) or median and interquartile ranges (IQRs) according to their distribution. Kruskal–Wallis, Mann–Whitney U, and chi-squared tests were performed to compare baseline characteristics among the groups, as appropriate.
The variable “time from first positive to negative nasopharyngeal swab” was dichotomized in ≤ 10 and > 10 days and the variable “age” in ≤ 80 and > 80 years according to Classification and Regression Tree analysis.
According to the primary outcome, to explore differences among the three groups (molnupiravir, nirmatrelvir/ritonavir, early-remdesivir) a one-way analysis of variance (ANOVA) was performed, with p-values adjusted using the Scheffé correction and the Bonferroni method for multiple comparisons.
Then, a multivariable Cox regression analysis was performed to identify factors independently associated with the composite endpoint using a forward stepwise procedure. Variables with statistical significance in the univariate analysis (p < 0.05) and those deemed of clinical relevance were entered in the multivariate model. Variables included for significance in univariate analysis included: age ≤ 80 years old, presence of three or more comorbidities, time from start of symptoms to antiviral treatment, cerebrovascular disease, hematological disease, chronic liver disease, autoimmune disease, presence of cough and gastrointestinal symptoms, and antiviral; variables included for clinical relevance included: chronic lung disease, immunosuppression, and adequate COVID-19 vaccination. The variable “antiviral treatments” was categorized into three categories: remdesivir (entered as reference variable), nirmatrelvir/ritonavir, and molnupiravir. Since antiviral treatments might not be started with the same timing from symptoms onset, the variable “antiviral treatments” was modeled as a time-dependent variable. More specifically, the variable “T_COV_” was created as a function of the time variable (time from symptoms onset to antiviral administration) “T_” and the covariate in question (type of antiviral), and has been included as a covariate in the Cox Regression model [17].
A post-hoc power analysis was performed based on proportions of death or hospitalization reported in the three randomized controlled trials in patients treated with molnupiravir (7.3%), nirmatrelvir/ritonavir (0.77%), and remdesivir (0.7%), respectively [7,8,9]. Our study has a power of 87.5% (α 0.05) to identify differences between molnupiravir and nirmatrelvir/ritonavir and 84.8% (α 0.05) to highlight differences between molnupiravir and remdesivir. Conversely, our sample is underpowered to identify potential differences between remdesivir and nirmatrelvir/ritonavir.
According to the secondary outcome, we described the proportion of AEs in the three groups. A multivariable Cox regression analysis was performed to identify factors independently associated with the occurrence of a negative nasopharyngeal swabs within 10 days from the first positive swab using the same methodology. This outcome was also explored in three further comparisons: nirmatrelvir/ritonavir versus molnupiravir, nirmatrelvir/ritonavir versus remdesivir, and molnupiravir versus remdesivir.
Results are expressed as the hazard ratio (HR) and their 95% CIs. Values of p < 0.05 were considered statistically significant. Statistical analyses were performed with the SPSS, version 27.0 (IBM, Armonk, NY, USA).
Results
Study Population Characteristics
A total of 562 outpatients with COVID-19 were included: 114 (20.3%) received molnupiravir, 252 (44.8%) nirmatrelvir/ritonavir, and 196 (34.9%) remdesivir. The median age was 69 (55–78.25) years old and 455 (81%) patients had adequate COVID-19 vaccination. Comparison of patients in the three treatment groups is presented in Table 2. Patients who received remdesivir were older, more frequently had more than two comorbidities, were more frequently affected by cardiovascular disease, and were more commonly solid organ transplant recipients compared with patients in the orally administered antiviral treatment groups. The time from symptoms onset to antiviral treatment was longer in the remdesivir (4 days, IQR 3–5 days) than in molnupiravir or nirmatrelvir/ritonavir group (3 days, IQR 2–4 days, p < 0.001). Adequate COVID-19 vaccination status was higher in patients treated with nirmltrevir/ritonavir.
Primary Outcome
Overall, the composite endpoint of death or hospitalization due to COVID-19 occurred in 14/562 (2.5%) patients. This event occurred more frequently in patients treated with remdesivir (n = 10/196, 5.1%) compared with those who received molnupiravir (n = 2/114, 1.8%) or nirmatrelvir/ritonavir (n = 2/252, 0.8%, difference among group p = 0.012). Adjustment for multiple comparison showed that this difference was statistically significant comparing nirmatrelvir/ritonavir versus remdesivir, but not comparing molnupiravir versus remdesivir or nirmatrelvir/ritonavir versus molnupiravir (Table 2).
With respect to patients who achieved clinical cure, patients who met the composite endpoint were older, more frequently had more than three comorbidities, were more likely to be affected by cerebrovascular disease, hematological disease, chronic liver disease, and autoimmune disease, and had a longer time from symptom onset to antiviral treatment (Supplementary Material, Table S1). Patients who died or required hospitalization more frequently received remdesivir (n = 10/14, 71.4%) compared with those who did not (n = 186/548, 33.9%, p = 0.01). On multivariable Cox regression analysis, the presence of three or more comorbidities (HR 6.89, 95% CI 1.52–31.14, p = 0.012), hematological disease (HR 5.59, 95% CI 5.59, 95% CI 1.84–16.94, p = 0.002), gastrointestinal symptoms (HR 3.87, 95% CI 1.27–11.81), and each-day increment from symptom onset to antiviral treatments (HR 1.54, 95% CI 1.03–2.28, p = 0.034) were factors independently associated with death or need for hospitalization due to COVID-19 (Table 3). The type of antiviral treatment was not independently associated with the risk of death or hospitalization (nirmatrelvir/ritonavir HR 1.46, 95% CI 0.21–9.92, p = 0.7; molnupiravir HR 2.68, 95% CI 0.4–17.95, p = 0.311; remdesivir as reference variable).
Secondary Outcomes
Data about safety were recorded in 541 patients who completed the safety questionnaire (safety evaluable population). As presented in Table 4, AEs occurred more frequently in the nirmatrelvir/ritonavir (n = 116/236, 49.2%) group compared with the molnupiravir (n = 23/109, 21.1%) and remdesivir groups (9/196, 4.6%, p < 0.001).
Among patients who received nirmatrelvir/ritonavir, the most common AE was dysgeusia (n = 99/236, 41.9%), followed by gastrointestinal disorders (n = 23/236, 9.7%) and headache (n = 3/236, 1.3%); two patients (0.8%) had hypotension because of concomitant antihypertensive drugs that were not discontinued and one patient (0.4%) complained of insomnia. Among patients treated with molnupiravir, the most common AEs were gastrointestinal disorders (n = 14/109,12.8%). Other AEs were rare and represented by skin rash (n = 3/109, 2.8%), dysgeusia (n = 3/109, 2.8%), headache (n = 1/109, 0.9%), and insomnia (n = 1/109, 0.9%). One patient had transient leukopenia and one had a slight increase in their transaminases values. In patients treated with remdesivir, AEs were very rare and mainly represented by asymptomatic bradycardia (n = 7/196, 3.6%).
Discontinuation because of an AE was uncommon in the three study groups (molnupiravir 4/109, 3.7%; nirmatrelvir/ritonavir 5/236, 2.1%; remdesivir none). Overall, 7 (1.3%) patients reported a rebound of symptoms after the complete course of antivirals: 2/109 (1.8%) and 5/236 (2.1%) in the nirmatrelvir/ritonavir and molnupiravir group, respectively (p = NS).
Negative swab within 10 days from the first positive swab was more common in patients who received nirmatrelvir/ritonavir (n = 104/252, 41.3%) compared with molnupiravir (n = 30/114, n = 26.3%) and remdesivir (n = 40/196, 20.4%, difference among groups p < 0.001).
Comparison of patients who had a negative swab ≤ 10 days from the first positive one and those who did not is reported in Supplementary Material, Table S2. On multivariable Cox regression analysis (Table 5), adequate COVID-19 vaccination (HR 1.53, 95% CI 1.04–2.23, p = 0.03), age < 80 years old (HR 1.8, 95% CI 1.23–2.6, p = 0.003), and nirmatrelvir/ritonavir compared with remdesivir (HR 1.73, 95% CI 1.25–2.4, p < 0.001) were factors independently associated with increased probability of having a negative nasopharyngeal swab within 10 days from the first positive swab. Each-day increase from symptoms onset to antiviral treatment was associated with a reduced chance of having a negative swab within 10 days from the first positive one (HR 0.88, 95% CI 0.78–0.98).
Subgroup analysis revealed that nirmatrelvir/ritonavir was independently associated with increased probability of having a negative swab within 10 days from the first one compared with remdesivir, also in the subgroup of patients with immunosuppression, more than two comorbidities, adequate vaccination status, and subjects more than 65 years old (Fig. 1). Comparing patients who received nirmatrelvir/ritonavir to those who received molnupiravir, there was no difference in the probability of having a negative swab within 10 days, with exception of the subgroup of patients with more than two comorbidities and those more than 65 years old (Supplementary Material, Fig. S1). Comparison of molnupiravir and remdesivir revealed no differences in achieving this outcome, except for patients with chronic kidney disease (Supplementary Material, Fig. S2).
Multivariable hazard ratios for factors associated with increased probability to have a negative nasopharyngeal swab within 10 days from the first positive one in patients treated with nirmatrelvir/ritonavir versus those treated with remdesivir. The multivariable model was adjusted for age < 80 years, adequate COVID-19 vaccination, ≥ two comorbidities, hypertension, time from symptom onset, hematological malignancy, immunosuppression, and chronic lung disease. Antiviral treatments were modeled as a time-dependent variable
Discussion
In this real-world experience of outpatient management of COVID-19, the risk of progression (death or hospitalization) did not differ among patients treated with molnupiravir, nirmatrelvir/ritonavir, or 3-day remdesivir. In this cohort of high-risk subjects, we observed that only 2.5% of treated patients died or were hospitalized due to COVID-19. Although nirmatrelvir/ritonavir is associated with a faster negative nasopharyngeal swab, it is also associated with a higher incidence of AEs, mainly represented by dysgeusia.
Notably, this study was conducted while Omicron was the dominant variant. A recent comparative analysis showed that Omicron is associated with a reduced risk of hospitalization and death compared with the Delta variant, but also highlighted a considerable variation in the severity according to age [18]. In fact, among subjects infected with the Omicron variant, hospital admission up to 14 days after a positive test and 28-day mortality were 11.1% and 5.12% in patients ≥ 80 years, respectively [19]. Similarly, other studies reported that the risk of hospitalization in Omicron-infected subjects ranged from 1% to 5% [20, 21], but these data are not stratified according to individual risk factors for progression or receipt of antiviral treatments. Notably, the proportion of death or hospitalization due to COVID-19 in our cohort of patients (2.5%) was low considering that the median age was 69 years, that a quarter of patients had immunosuppressive state, and 11% were affected by solid or hematological malignancy.
In this real-world experience, there was no statistically significant difference in the risk of COVID-19 progression in the three treatment groups. However, we highlighted that the choice of antivirals should be individualized according to patients’ comorbidities and characteristics. Nirmatrelvir/ritonavir have interactions with other drugs and should be avoided in some categories of patients, such as solid organ transplant recipients who received chronic immunosuppressive therapy, such as tacrolimus or mycophenolate, for whom other alternative antivirals might be appropriate. The use of oral antivirals is easier with respect to 3-day iv remdesivir. Early use of remdesivir is associated with reduced risk of disease progression [22], but a dedicated service equipped with nursing staff is needed for outpatients. Moreover, although the oral administration is easier to handle, it should be underlined that oral antivirals, especially nirmatrelvir/ritonavir, are not free from AEs. We found that almost half of patients treated with nirmatrelvir/ritonavir developed an AE, mainly represented by dysgeusia. Although AEs were usually mild, dysgeusia and gastrointestinal symptoms may be disabling for COVID-19 patients, especially for elderly and frail ones. Thus, patients who received nirmatrelvir/ritonavir should be carefully screened through an evaluation of baseline renal function and exclusion of drug–drug interactions, and the decision to discontinue some drugs during the course of therapy as well as monitoring potential AEs is essential. Thus, implementing ambulatory services equipped with Infectious Diseases (ID) specialists caring for outpatients with COVID-19 may represent a promising strategy to reduce hospitalizations due to COVID-19 and improve patient care and safety.
We found that patients treated with nirmatrelvir/ritonavir had a higher probability of having a negative test within 10 days from the first positive one. This data may be affected by some study limitations: this is not an interventional study and patients did not receive a nasopharyngeal swab every day. However, this happened in all three study groups. This endpoint may be particularly important for some patients, such as onco-hematological patients who wait for a negative test to start chemotherapy or other interventions, and patients who need negative test to undergo diagnostic procedures for other underlying diseases. This advantage of nirmatrelvir/ritonavir remained also after adjustment for relevant confounding factors, such as adequate COVID-19 vaccination status, and in specific subgroups, such as patients with immunosuppressive status and elderly patients. This advantage disappears when we compared nirmatrelvir/ritonavir with molnupiravir.
Finally, we found that about 2% of patients treated with nirmatrelvir/ritonavir and molnupiravir experienced a rebound of symptoms after the antiviral discontinuation. Here, we only documented rebound of symptoms and were not able to perform virological study on these cases. Cases of recrudescence of SARS-CoV-2 infection after nirmatrelvir/ritonavir treatment are being increasingly reported [23, 24]. However, it is not known if rebound may occur in the general population of infected patients or whether is unique to nirmatrelvir/ritonavir. A large study available as a preprint showed that there are no significant differences in COVID-19 rebound risks between nirmatrelvir/ritonavir and molnupiravir, while patients who experience rebound had significantly higher prevalence of underlying medical conditions than those without [25]. The phenomenon of COVID-19 rebound associated with specific antiviral treatments should be further investigated. However, the risk of rebound should not represent an obstacle to antiviral treatments.
This study has several limitations. It is an observational study and allocation bias may occur among the three study groups. Although in the analysis we took into account a large number of confounders associated with high risk for severe COVID-19, residual confounding among the three groups remains of concern. We did not perform a systematic identification of the SARS-CoV-2 variant. However, this study was conducted during the spread of Omicron variant in Italy. We were not able to perform daily nasopharyngeal swab to assess negative test results. However, our study design well reflects the real-world management of COVID-19 patients. Finally, since this is a real-world experience, we included all consecutive patients admitted to our outpatient clinic during the study period and the sample size was not calculated a priori. However, we performed a post-hoc power analysis [26]. Our sample has a power higher than 80% to identify differences between molnupiravir versus nirmatrelvir/ritonavir and between molnupiravir versus remdesivir, but it is underpowered to evaluate differences between nirmatrelvir/ritonavir versus remdesivir. Further studies are needed to highlight differences between these latter antivirals, especially in patients with immunosuppression.
Conclusions
We reported a real-world experience of outpatients management of COVID-19 in subjects at high risk of progression treated with the three available antivirals: nirmatrelvir/ritonavir, molnupiravir, and remdesivir. Despite the limitations related to its observational nature and statistical power, our study detected no differences in the risk of COVID-19 progression among the three treatments. Patients treated with nirmatrelvir/ritonavir more frequently reported mild AEs, but had a higher probability of having a negative test within 10 days from the SARS-CoV2 infection. An early individualized outpatient treatment, taking into consideration timing of symptom onset, potential drug–drug interactions, and underlying diseases may represent the cornerstone of COVID-19 management in the Omicron era.
References
Tiseo G, Yahav D, Paul M, Tinelli M, Gavazzi G, Mussini C, et al. What have we learned from the first to the second wave of COVID-19 pandemic? An international survey from the ESCMID Study Group for Infection in the Elderly (ESGIE) group. Eur J Clin Microbiol Infect Dis. 2022;41:281–8.
Menichetti F, Popoli P, Puopolo M, Spila Alegiani S, Tiseo G, et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4:e2136246 (Erratum in: JAMA Netw Open. 2022;5:e2146944).
Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382–93.
Falcone M, Tiseo G, Valoriani B, Barbieri C, Occhineri S, Mazzetti P, et al. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther. 2021;10:2479–88.
Yamasoba D, Kosugi Y, Kimura I, Fujita S, Uriu K, Ito J, Sato K, Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect Dis. 2022;22:942–3.
Zhou H, Tada T, Dcosta BM, Landau NR. Neutralization of SARS-CoV-2 omicron BA.2 by therapeutic monoclonal antibodies. bioRxiv [Preprint]. 2022. https://doi.org/10.1101/2022.02.15.480166 (Update in: Viruses 14(6)).
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–15.
Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–7.
Mahase E. Covid-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;375: n2422.
NIH COVID-19 Treatmment Guidelines. Last Updated: September 26, 2022. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
Lamb YN. Nirmatrelvir plus ritonavir: first approval. Drugs. 2022;82:585–91 (Erratum in: Drugs 82:1025).
Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740–6.
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis 2022;ciac443
Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563.
Munoz-Price LS, Frencken JF, Tarima S, Bonten M. Handling time-dependent variables: antibiotics and antibiotic resistance. Clin Infect Dis. 2016;62:1558–63.
Prendki V, Tiseo G, Falcone M, ESCMID Study Group for Infections in the Elderly (ESGIE). Caring for older adults during the COVID-19 pandemic. Clin Microbiol Infect. 2022;28:785–91.
Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–12.
Ridgway JP, Tideman S, Wright B, Robicsek A. Decreased Risk of Coronavirus Disease 2019–related hospitalization associated with the omicron variant of Severe Acute Respiratory Syndrome Coronavirus 2. Open Forum Infect Dis 2022;ofac288.
Bager P, Wohlfahrt J, Bhatt S, Stegger M, Legarth R, Møller CH, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22:967–76.
Falcone M, Suardi LR, Tiseo G, Barbieri C, Giusti L, Galfo V, et al. Early use of remdesivir and risk of disease progression in hospitalized patients with mild to moderate COVID-19. Clin Ther. 2022;44:364–73.
Carlin AF, Clark AE, Chaillon A, Garretson AF, Bray W, Porrachia M, et al. Virologic and immunologic characterization of COVID-19 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis 2022;ciac496
Rubin R. From Positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take paxlovid. JAMA. 2022;327:2380–2.
Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID-19 rebound after paxlovid and molnupiravir during January-June. medRxiv 2022 Jun 22:2022.06.21.22276724 [Preprint].
Shreffler J, Huecker MR. Type I and Type II Errors and Statistical Power. 2022 Mar 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32491462.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the University of Pisa.
Author Contributions
Concept and design: Giusy Tiseo and Marco Falcone; Conducting the research and data collection: Chiara Barbieri, Valentina Galfo, Sara Occhineri, Tommaso Matucci, Francesco Almerigogna, Jona Kalo, Pietro Sponga, Mario Cesaretti, Gabriele Marchetti, Arianna Forniti, Claudio Caroselli, Simone Ferranti, Manuela Pogliaghi, Marina Polidori, Silvia Fabiani, Stefano Verdenelli, Enrico Tagliaferri, Niccolò Riccardi, Lorenzo Roberto Suardi, Claudia Carmignani, Serena Batini, Luca Puccetti, Riccardo Iapoce; Drafting of the manuscript: Giusy Tiseo; Statistical analysis: Giusy Tiseo; Critical revision of the manuscript for important intellectual content: Francesco Menichetti and Marco Falcone. Visualization, Validation and final approval: all the authors.
Disclosures
Giusy Tiseo received honoraria for educational meetings by Shionogi. Francesco Menichetti has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, Merck Sharp & Dohme (MSD), Nordic Pharma, Pfizer, Astellas, Gilead, Bristol-Myers Squibb (BMS), Janssen, ViiV, bioMérieux, Biotest, Becton Dickinson, Pfizer, and Shionogi. Marco Falcone received unconditional grants/or speaker honoraria from MSD, Angelini, Shionogi, Pfizer, Menarini, TermoFisher, Gilead and Nordic Pharma. Declared conflicts of interest are outside the submitted work and did not affect the scientific objectivity of this study. Chiara Barbieri, Valentina Galfo, Sara Occhineri, Tommaso Matucci, Francesco Almerigogna, Jona Kalo, Pietro Sponga, Mario Cesaretti, Gabriele Marchetti, Arianna Forniti, Claudio Caroselli, Simone Ferranti, Manuela Pogliaghi, Marina Polidori, Silvia Fabiani, Stefano Verdenelli, Enrico Tagliaferri, Niccolò Riccardi, Lorenzo Roberto Suardi, Claudia Carmignani, Serena Batini, Luca Puccetti and Riccardo Iapoce have nothing to declare.
Compliance with Ethics Guidelines
All patients signed a written informed consent. The study was conducted according to the principles stated in the Declaration of Helsinki, and approved by the local Ethical Committee of the Area Vasta Nord Ovest of Tuscany region (IRB number 230320).
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Corresponding author Prof. Marco Falcone, Department of Clinical and Experimental Medicine, University of Pisa, Italy, Via Paradisa 2, 56124, Pisa, Italy, marco.falcone@unipi.it. Alternate corresponding author Dr Giusy Tiseo, Department of Clinical and Experimental Medicine, University of Pisa, Italy.”
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tiseo, G., Barbieri, C., Galfo, V. et al. Efficacy and Safety of Nirmatrelvir/Ritonavir, Molnupiravir, and Remdesivir in a Real-World Cohort of Outpatients with COVID-19 at High Risk of Progression: The PISA Outpatient Clinic Experience. Infect Dis Ther 12, 257–271 (2023). https://doi.org/10.1007/s40121-022-00729-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00729-2