Abstract
Introduction
Clostridioides difficile infection (CDI) is a major public health threat. Up to 40% of patients with CDI experience recurrent CDI (rCDI), which is associated with increased morbidity. This study aimed to define an at-risk population by obtaining a detailed understanding of the different factors leading to CDI, rCDI, and CDI-related morbidity and of time to CDI.
Methods
We conducted a systematic literature review (SLR) of MEDLINE (using PubMed) and EMBASE for relevant articles published between January 1, 2016, and November 11, 2022, covering the US population.
Results
Of the 1324 articles identified, 151 met prespecified inclusion criteria. Advanced patient age was a likely risk factor for primary CDI within a general population, with significant risk estimates identified in nine of 10 studies. Older age was less important in specific populations with comorbidities usually diagnosed at earlier age, such as bowel disease and cancer. In terms of comorbidities, the established factors of infection, kidney disease, liver disease, cardiovascular disease, and bowel disease along with several new factors (including anemia, fluid and electrolyte disorders, and coagulation disorders) were likely risk factors for primary CDI. Data on diabetes, cancer, and obesity were mixed. Other primary CDI risk factors were antibiotics, proton pump inhibitors, female sex, prior hospitalization, and the length of stay in hospital. Similar factors were identified for rCDI, but evidence was limited. Older age was a likely risk factor for mortality. Timing of primary CDI varied depending on the population: 2–3 weeks in patients receiving stem cell transplants, within 3 weeks for patients undergoing surgery, and generally more than 3 weeks following solid organ transplant.
Conclusion
This SLR uses recent evidence to define the most important factors associated with CDI, confirming those that are well established and highlighting new ones that could help to identify patient populations at high risk.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study suggests that older age is a risk factor for Clostridioides difficile infection (CDI) in the general population. |
Data confirm the established risk factor status of antibiotics, as well as certain comorbidities (kidney disease, liver disease, cardiovascular disease, and infection). |
The results also highlight potential new risk factors, including anemia, coagulation, fluid and electrolyte disorders, and being underweight. |
Older age, antibiotic intake, and hospitalization are risk factors for mortality. |
The time to CDI onset varies and depends on the patient cohort. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24975549.
Introduction
Clostridioides difficile, previously known as Clostridium difficile, is a major public health threat and one of the most common healthcare-associated infections globally [1, 2]. It is a Gram-positive, anaerobic, toxin-producing bacillus, the spores of which are transmitted by the fecal–oral route and can survive in the environment for several months [1, 2]. The clinical presentation of C. difficile infection (CDI) ranges from asymptomatic carriage to mild or moderate diarrhea, and to severe and potentially fatal pseudomembranous colitis, toxic megacolon, and sepsis [2]. Hospitalization occurs within 6 days of specimen collection in 42% of cases, and in-hospital death occurs in 2% of cases [3]. Toxic megacolon or pseudomembranous colitis occur in fewer than 1% of cases [3]. Approximately 5–15% of healthy adults [1, 4], up to 70% of newborns and healthy infants [1], and 57% of long-term care facility residents are colonized by C. difficile [4]. In the USA, there are nearly half a million cases of CDI and approximately 30,000 deaths annually [5, 6]. Most cases and deaths occur in individuals aged ≥ 65 years, although an estimated 27% of cases and 17% of deaths occur in the 45–64-year age group [6].
The proportion of community-derived CDI has increased, accounting for approximately one-third of cases in 2011 [5], and 50% of cases in 2018 [7]. However, the rate of CDI- and recurrent CDI (rCDI)-related deaths remains higher for healthcare-acquired rather than community-acquired disease [5, 6]. Recovery from CDI is complicated by recurrence, which affects approximately 15–35% of individuals [8,9,10]. The Centers for Disease Control and Prevention (CDC) defines rCDI as CDI cases with a positive C. difficile stool specimen between 2 and 8 weeks after the last positive specimen [11]. Approximately 40–60% of individuals with an initial recurrence have a second recurrence [12], and recurrences are associated with increased morbidity [10].
Risk factors for CDI can be divided into three general categories: host factors (advanced age, impaired immune status, and comorbidities); increased risk of exposure to C. difficile spores (longer length of hospital stays, healthcare environment, infected roommates, or hand carriage by personnel); and factors that disrupt the normally protective gut microbiota (antimicrobials, other medications, or procedures) [13].
Nearly every antibiotic has been associated with the development of CDI, including antibiotics used for CDI treatment, leading to many patients developing recurrent disease despite repeated antibiotic courses [1]. Broad-spectrum penicillins and cephalosporins, clindamycin, and fluoroquinolones have been associated with a higher risk of inducing CDI than other antibiotics [1, 14, 15]. In addition, the antibiotics used to treat CDI are associated with different CDI recurrence rates, with lower rates seen with vancomycin versus metronidazole [16] and with fidaxomicin versus vancomycin [17]. CDI risk is highest during systemic antimicrobial therapy and in the first month following cessation of antimicrobial therapy [1]. Factors can also predispose individuals to single or multiple recurrences of CDI [18, 19]; such risk factors include advanced patient age, prior antibiotic use, gastric acid suppression, infection with a hypervirulent strain, use of antibiotics following completion of CDI treatment, and length of hospital stay [20,21,22,23].
Considering the high recurrence rate, prevention of CDI and rCDI is a key clinical issue. Although current treatment and preventive measures are limited, future measures could help to reduce CDI morbidity and mortality. For these measures to be effective and address the unmet needs of patients, evidence regarding risk factors is needed to enhance the understanding of the populations that may benefit the most. Although many studies have investigated the association between individual risk factors and the occurrence of CDI [24,25,26], studies are difficult to compare because of differences in design, outcomes, and populations.
We conducted a systematic literature review (SLR) of publications describing populations with CDI in the USA, published between January 1, 2016, and November 11, 2022. The aim of this SLR was to obtain a detailed understanding of the different risk factors leading to CDI, rCDI, and CDI-related morbidity and of the time to CDI.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The reporting of this SLR was guided by the standards in the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement [27].
Study Objectives
The primary objective was to establish the risk factors for CDI and identify at-risk subpopulations. The secondary objective was to gain more insight into the various risk factors and time to onset of CDI to identify specific stages of underlying disease (e.g., end-stage versus early-stage renal disease) that may increase CDI risk or serious CDI outcomes.
Identification of Studies
The core of the review was a literature search of MEDLINE using PubMed, supplemented with a search in EMBASE, for relevant articles published between January 1, 2016, and November 11, 2022. Search strings were developed that combined keywords and related terms for C. difficile, the USA, and study design (see “Supplementary Material: Search Strings”). Our search was restricted to US-based studies to limit data heterogeneity. Additionally, the USA is unique in terms of its size and data detail and availability.
Selection Procedure
From the articles retrieved from MEDLINE and EMBASE, the relevant references were selected by two authors independently, through a three-step selection process: screening of title and abstract; screening of full article; and data extraction. Inclusion criteria consisted of US-based studies; the presence of relevant data for objectives (risk factors, time between exposure and onset of disease); an adult (aged ≥ 18 years) study population; inclusion of risk factors presented with odds ratio (OR), hazard ratio (HR), or relative risk (RR). Articles that described non-pertinent publication types (e.g., letters to the editor, editorials, or comments) were excluded, as were case reports. Modeling studies that did not provide original data and C. difficile incidence or prevalence studies were also excluded. Further exclusion criteria were articles in which the methods section did not provide sufficient detail to understand what had been done and articles for which quantitative data could not be retrieved. For articles presenting similar results from identical datasets, only one was included. In the case of meta-analyses or systematic reviews, the reference list was checked for missed relevant articles; in such cases, the original articles were included and the systematic review or meta-analysis itself was excluded. See “Supplementary Material: Screening” for further details on the screening process.
Study Quality Assessment Checklist and Procedure
Scottish Intercollegiate Guidelines Network (SIGN) methodology checklists were used to rate the quality of the studies: high quality (++), the majority of the criteria of the SIGN checklist were met—there was little or no risk of bias and the results were unlikely to be changed by further research; acceptable (+), most criteria were met—some flaws in the study were present with an associated risk of bias and conclusions may change in the light of further studies; low (−), either most criteria were not met or there were significant flaws in key aspects of the study design.
Important quality aspects were retrospective or prospective design; sample size; description of the study population and main characteristics; description of statistical models and appropriate adjustments of the models or appropriate matching of cases and controls; number of variables in the final statistical model in relation to the sample size; clear definitions of the outcomes; setting of the study (e.g., single center or database review); and representativeness of the study population. The SIGN score was added to the data-extraction sheet. Data extraction was performed in Excel. See “Supplementary Materials: Data Extraction” for details on what was included and decisions that were made while compiling the data-extraction tables.
Quality Control
All titles and abstracts were screened by two independent researchers. The results were compared, and deviations were discussed. In the event of doubt by both researchers, the title was selected for full-text evaluation. The first 10% of full-text articles were checked for relevancy in duplicate by two independent researchers. The results were compared and discussed early in the process. All doubts and exclusions were discussed. For data extraction, all evidence tables were compiled by senior researchers (EB, JE) and reviewed by a second researcher (EB, JE, or ESU).
Results
Study Characteristics and Outcomes
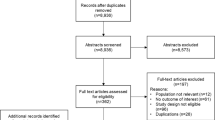
Our search yielded 1324 articles, of which 151 met prespecified inclusion criteria (Fig. 1). Most studies were retrospective (n = 115). Other studies were of case–control/case–cohort (n = 26), prospective cohort (n = 7), or nested case–control design (n = 2). For one study, it was not clear if the design was retrospective or prospective [28]. No articles were included from manual searches of the reference lists of meta-analyses or systematic reviews.
Risk Factors for CDI
Risk estimates for possible risk factors and the development of CDI were presented in 101 studies.
Age, Sex, and Lifestyle
Age was reported in 99 studies, with 46 including a risk estimate for age as a possible CDI risk factor (Fig. 2). In general populations, older age was significantly associated with CDI in nine of 10 studies [29,30,31,32,33,34,35,36,37,38]. For example, in the large sample of hospitalized patients included in the multicenter study by Webb et al., older age was significantly associated with CDI (adjusted OR [aOR] per year of increase 1.01, 95% confidence interval [CI] 1.006–1.013); p < 0.0001) [38]. However, in specific patient populations, age was often not related to CDI risk. For example, age was a significant risk factor in only two of four studies in patients receiving solid organ transplants [28, 39,40,41], one of three studies in patients with bowel disease [42,43,44], and one of four studies in patients with cancer [45,46,47,48].
Age and gender as possible risk factors for CDI in patients from non-specific or general populations, patients undergoing surgery or transplants, patients with bowel disease or cancer, elderly patients, and othera patient groups. aOther populations: alcoholic hepatitis (n = 1); cardiac devices (n = 1); cirrhosis (n = 4; one study describes CDI in patients with cirrhosis and patients with hepatocellular carcinoma); Helicobacter pylori infection (n = 1); HIV (n = 1); intensive care unit (n = 1); kidney disease (n = 2); neurocritical illness (n = 1); patient population (UTI, pneumonia, or sepsis) (n = 1); pancreatitis (n = 2); pregnant women (n = 2); spinal cord injury (n = 1); UTI (n = 1); pharyngitis, sinusitis, influenza, viral upper respiratory infection, non-suppurative otitis media, bronchiolitis, monomicrobial bloodstream infections (n = 1). CDI Clostridioides difficile infection, Nr number, pop population, transpl-cells hematopoietic stem cell transplantation, transpl-organs organ transplantation, UTI urinary tract infection
Twenty-six articles included a risk estimate for sex (Fig. 2); a significantly higher risk of CDI in women compared with men was reported in 12 studies [28, 43, 47,48,49,50,51,52,53,54,55]. This ranged from an aOR of 1.02 (95% CI 1.00–1.04; p value not presented) in a mixed study population in a large database study [34] to an aOR of 6.3 (95% CI 1.7–24.0; p < 0.01) in patients with urinary tract infections (UTIs) [50]. Risk was higher for men than women in one study (aOR 1.47, 95% CI 1.22–1.80; p = 0.0001) [56].
Data regarding the risk associated with current or history of smoking or alcohol or drug use were inconclusive. Smoking was not associated with CDI risk in a study of patients with neurosurgical trauma by Belton et al., (aOR 1.246, 95% CI 0.797–1.949; p = 0.334) [57]. In contrast, in the study by Gonzalez et al., patients with orthopedic surgery had an increased risk of CDI with a history of smoking (aOR 1.42, 95% CI 1.14–1.77; p = 0.002) [58].
Comorbidities
Table 1 provides a summary of comorbidities as potential risk factors for CDI. Fifteen of 18 studies identified a significant risk estimate for CDI with kidney disease (Supplementary Table S1). Two large studies of a general hospital population with renal failure reported aORs of 1.58 (95% CI 1.54–1.62) and 1.93 (95% CI 1.40–2.65) [31, 34]. A hospitalized patient population with acute renal failure had aORs of 4.88 (95% CI 4.66–5.12) for age 50–64 years and 4.43 (95% CI 4.24–4.62) for age ≥ 65 years [56]. In addition, biomarkers related to poor kidney function had risk estimates significantly associated with CDI in four of six studies, with aORs of 1.43 (96% CI 1.30–1.57) [52], 1.25 (95% CI 1.11–1.43) [59], and 1.004 (95% CI 1.001–1.007) [38] and an adjusted HR (aHR) of 1.09 (95% CI 1.02–1.16) [60] (Supplementary Table S1). Furthermore, five of seven studies found that dialysis increased the risk of CDI [35, 52, 56, 58, 60,61,62] (Supplementary Table S1). Most studies were in surgical patients, and the largest of these reported an aOR of 1.21 (95% CI 1.05–1.39) [52].
Eight of 10 studies of patients with liver disease reported the disease to be a risk factor for CDI (Supplementary Table S2) [34, 38, 47,48,49, 55, 63,64,65,66]. This included general hospital populations with liver disease, with aORs of 1.34 (95% CI 1.29–1.40) [34] and 1.30 (95% CI 1.1–1.4) [38]. Hepatic encephalopathy appeared to have a protective effect against CDI in patients with cirrhosis (aOR 0.86, 95% CI 0.82–0.90) or hepatocellular carcinoma (aOR 0.75, 95% CI 0.60–0.93) [47]. Liver disease (among other pathophysiologic states) is often associated with changes in serum albumin [67, 68], and low albumin levels were significantly associated with an increased risk of CDI among surgery patients in four of five studies, with aORs of 1.44 (95% CI 1.19–1.74) and 1.41 (95% CI 1.17–1.69), 1.48 (95% CI 1.37–1.59) [52], 3.15 (95% CI 1.25–6.84) [63], and 6.4 (95% CI 2.49–16.43) [63] (Supplementary Table S2).
Nine studies reported that various cardiovascular diseases may be risk factors for CDI; however, four found a protective effect (Supplementary Table S3) [34, 35, 42, 48, 49, 59, 63,64,65, 69,70,71,72]. Results differed depending on the specific population and cardiovascular disease. For example, congestive heart disease had a protective effect in patients with ischemic colitis or diverticulitis (aOR 0.84, 95% CI 0.76–0.94) [42] but was a risk factor in patients undergoing neurologic surgery (aOR 2.79, 95% CI 1.82–4.28) [70] and in a general hospital population (aOR 1.622, 95% CI 1.116–2.357) [35]. Hypertension was investigated separately, with mixed results from 13 studies. For example, it was found to be a risk factor in three of the six studies in surgery patients (aHR 2.51, 95% CI 1.06–5.98 [73]; aOR 1.55, 95% CI 1.12–2.18 [63]; and aOR 1.09, 95% CI 1.02–1.16 [52]), but it had a protective effect in four studies with various populations (aOR 0.85, 95% CI 0.83–0.87 [65]; aOR 0.82, 95% CI 0.75–0.89 [48]; aOR 0.75, 95% CI 0.73–0.76 [34]; and aOR 0.55, 95% CI 0.34–0.90 [31]) (Supplementary Table S4).
Risk estimates for a wide variety of infections were presented in 18 studies and are summarized in Supplementary Table S5. UTI and pyelonephritis were significant risk factors in five of seven studies. Sepsis and sepsis-related syndromes were significant risk factors for CDI in eight of 10 studies, pneumonia was a significant risk factor in seven of eight studies, and human immunodeficiency virus or acquired immunodeficiency syndrome was a risk factor for CDI in three of four studies. For example, hospital patients aged 50–64 years had an aOR of 4.32 (95% CI 4.15–4.50) with a UTI, 5.9 (95% CI 5.53–6.30) with sepsis, and 4.49 (95% CI 4.30–4.70) with pneumonia [56]. Notably, antibiotic use during infections may be related to the increased risk of CDI, as discussed in Sect. “Antibiotic Use”.
Anemia and low hematocrit were reported as risk factors for CDI in 11 of 13 studies. Six of these studies included patients undergoing surgery, with five reporting a significant risk: one large study of surgery patients with low hematocrit reported an aOR of 1.39 (95% CI 1.31–1.48) [52]. Five of six studies found fluid and electrolyte disorder to strongly increase the risk of CDI (aOR range 1.46 [95% CI 1.17–1.84] to 9.96 [95% CI 9.37–10.60]) [30, 31, 34, 42, 64, 70]. Bowel disease [30, 42,43,44, 48,49,50,51, 69, 74,75,76,77], coagulation disorders [31, 34, 42, 47, 49, 52, 54, 62, 64, 70], and being underweight [31, 32, 34, 42, 44, 49, 52, 64, 78, 79] were also found to be possible risk factors. Obesity appeared to have a protective effect and decreased the risk of CDI in four studies, with aORs ranging from 0.252 (95% CI 0.058–0.945) to 0.81 (95% CI 0.74–0.87) [34, 49, 52, 80], although no significant effect was found in the other seven studies [42, 48, 53, 63, 64, 78, 81]. From 15 studies on solid tumors, five reported cancer to be a significant risk factor and one reported a protective effect. However, six of seven studies on hematologic malignancies reported a significant risk for CDI, the aOR ranging from 1.74 (95% CI 1.48–2.06) to 12.9 (95% CI 3.7–44.8) [31, 34, 38, 64, 82,83,84]. Among six studies of metastatic cancer, significant risk was reported in three, and one reported a protective effect [34, 54, 62, 64, 82, 85]. Diabetes was reported in 15 studies, with four reports of significantly increased risk and six reports of a protective effect. Results for lung disease were also mixed: from 14 studies, five reported a significant risk and three reported a protective effect.
Antibiotic Use
A total of 46 studies with a CDI outcome reported a risk estimate for antibiotic use, and 27 of these evaluated general antibiotic use. Twenty of 27 studies found that general use of antibiotics significantly increased the risk of CDI. For example, in the large multicenter study by Webb et al., an increasing number of antibiotic days of therapy during admission was associated with CDI (aOR per day increase 1.007, 95% CI 1.005–1.009; p < 0.0001) [38]. Moreover, in the same study, an increasing number of antibiotic days of therapy in the prior 60 days was associated with CDI (aOR per day increase 1.128, 95% CI 1.122–1.134; p < 0.0001). However, five studies found no significant association. Previous SLRs and meta-analyses ranked the classes of antibiotics by their presumed CDI risk [86, 87]. We used these studies to categorize the reported antibiotics in our literature review into five groups: low risk, moderate risk, high risk, treatment for CDI, and not categorized (see Supplementary Table S6 for antibiotic categorization). High-risk antibiotics included cephalosporins, quinolones (fluoroquinolones), lincomycins, and carbapenems. As expected, CDI risk was increased with high-risk antibiotics in 17 of 22 studies (Fig. 3). In three studies, high-risk antibiotics (quinolones, clindamycin) had a protective effect and significantly decreased the risk of CDI [30, 88, 89].
Treatments
Eleven of 17 studies reported that proton pump inhibitors (PPIs) significantly increased the risk of CDI. In a general hospital population, PPI use was associated with aORs ranging from 1.32 (95% CI 1.00–1.73) to 2.48 (95% CI 2.15–2.85) [29, 31, 88, 90]. H2 antagonists were reported as significant risk factors in two of three studies, with aORs of 1.132 (95% CI 1.059–1.210) [88] and 1.45 (95% CI 1.11–1.91) [31]. In addition, increased risk of CDI was reported for studies of gastric acid suppression and for combined estimates for PPI and H2 antagonists, but not for antacids [38, 55, 88, 91]. Four of seven studies reported that steroid use significantly increased the risk of CDI; however, the evidence was limited [38, 52, 62, 80, 92,93,94]. CDI risk estimates related to surgery procedures or surgery outcomes were presented in 14 studies; wound infections or contaminated wounds were significant risk factors for CDI in two of the studies [52, 95]. Procedures considered to pose a higher risk of CDI included general surgery [52], circumferential surgery [70], and stoma reversal [62]. Risk factors were also investigated for patients undergoing transplantation. Eight of 14 studies reported significant risk factors related to transplantation, including presence of graft versus host disease and lung transplantation versus kidney transplantation.
Other Risk Factors
Prior hospitalization was identified as a significant risk factor for CDI in all 11 studies in which it was evaluated [35, 37, 38, 46, 51, 82, 84, 94, 96, 97]. In the large multicenter study by Webb et al., patients with a recent history of hospitalization (within the past 60 days) had an increased risk of CDI (aOR 2.2, 95% CI 1.5–3.4; p < 0.0001) [38]. In addition, length of stay (LOS) in hospital was presented in 16 studies: 10 identified LOS as a risk factor. In a study with acute and critical care surgery patients, increasing LOS was associated with CDI (aOR per day increase 1.013, 95% CI 1.003–1.024; p = 0.02) [98].
Other possible risk factors may include intensive care unit (ICU) stay [38, 41, 60, 99,100,101,102], emergency department stay [34, 69, 103], being a transfer patient [71], or being admitted from outside hospital or nursing/chronic care facilities [104]. However, these factors were described in different manners, and the small number of studies make conclusions difficult. Colonization with C. difficile was a highly significant risk factor for CDI in two studies (aOR 2.7, 95% CI 1.11–6.48; p = 0.025; aOR 13.52, 95% CI 3.46–52.83) [39, 105]. Two studies included pregnant women as a study population [75, 77]; in one study, multiple gestations and perineal or cesarean wound infections were significant risk factors for CDI (aOR 1.8, 95% CI 1.4–2.3; p < 0.001; and aOR 9.7, 95% CI 8.0–11.6; p < 0.001, respectively) [75].
rCDI Risk Factors
Data on risk of rCDI were presented in 30 studies and are summarized in Table 2. The definitions for rCDI varied across studies (Supplementary Table S7), as did the follow-up time from primary CDI to recurrence. Fifteen studies assessed the impact of age on the risk of rCDI, eight of which found a significantly increased risk; however, results were not consistent across all age groups. In the database study by Ma et al., age as a linear variable was associated with rCDI (aOR per 10-year increase 1.25, 95% CI 1.21–1.29; p < 0.001]) [106]. Many studies categorized patients in age groups. For example, in the study by Mathews et al., patients were categorized into the groups aged < 45 years, 45–54 years, 55–64 years, 65–74 years, and > 75 years [107]. The youngest group (aged < 45 years) was less likely to be readmitted compared with the oldest group (aged > 75 years) (aOR 0.828, 95% CI 0.79–0.86) [107]. In contrast, in one report, younger patients (aged 18–65 years) had a significantly higher risk of rCDI than older patients (aged > 65 years) [108].
Recurrence rate was not associated with sex in 10 of 12 studies [20, 44, 106, 108,109,110,111,112,113,114,115,116], though two studies reported an increased risk for women [106, 115]. In the large database study by Ma et al., women were at higher risk of rCDI compared with men (aOR 1.24, 95% CI 1.11–1.38; p < 0.001) [106]. In a small retrospective study by Benitez et al. [112], no difference was observed between men and women (aOR 1.02, 95% CI 0.71–1.47; p value not presented).
In eight of 15 studies on antibiotics, a significantly increased risk of rCDI was found. The classes or types of antibiotics used were compared in five of these studies [20, 111, 112, 117, 118]. Use of clindamycin, fluoroquinolones, third- or fourth-generation cephalosporins, ceftriaxone, or piperacillin/tazobactam prior to the initial CDI event were risk factors for rCDI. In the study by Appaneal et al., for example, the highest risk was observed with fluoroquinolones versus no use of fluoroquinolones (aOR 3.35, 95% CI 2.58–4.34; p value not presented) [117]. Increased risk was also found with aztreonam, penicillin/amoxicillin/ampicillin, or cephalosporins during an initial CDI event. For cephalosporins, Appaneal et al., reported an aOR of 1.92 (95% CI 1.13–3.25) with first- or second-generation cephalosporins [117]. Sutton et al., reported a higher risk of rCDI when primary CDI was treated with fidaxomicin compared with vancomycin or vancomycin combinations, although only a small proportion of patients were treated with fidaxomicin [111]. During their CDI treatment, patients receiving vancomycin had a decreased risk of developing rCDI in comparison with patients receiving fidaxomicin (aHR 0.335, 95% CI 0.204–0.548; p value not presented) [111]. In contrast, a large-scale study by Hall et al., found that rCDI was reduced in patients who were treated with fidaxomicin during initial CDI compared with vancomycin (aHR 0.67, 95% CI 0.50–0.90; p value not presented) [119]. Appaneal et al., further reported that the use of any antibiotic after completing primary CDI treatment was associated with an increased risk of rCDI (aOR 2.14, 95% CI 1.68–2.73; p value not presented) [117].
In four of eight studies, PPIs or acid-reducing medications significantly increased the risk of rCDI [20, 106, 109, 110, 113, 114, 117, 120]. In the study by Ma et al., PPI use ≤ 90 days before CDI was associated with rCDI (aOR 1.14, 95% CI 1.01–1.29; p = 0.039) [106]. Results for comorbidities are summarized in Supplementary Table S8. Increased risk of rCDI was reported in five of six studies on kidney disease [20, 106,107,108, 114, 115], four of five studies on liver disease [20, 108, 117, 121, 122], and both studies on fluid and electrolyte disorders [107, 108]. Reports on cardiovascular disease were less clear, with increased risk of rCDI seen with hypertension (aOR 1.17, 95% CI 1.15–1.20) [107], but heart failure has been associated with both increased (aOR 1.14, 95% CI 1.06–1.22) [115] and decreased risk (aOR 0.94, 95% CI 0.92–0.96) [107]. Three of five diabetes studies reported an increased risk of rCDI [20, 106,107,108, 114], with a peak OR of 1.36 (95% CI 1.14–1.67). Two of three lung disease studies [107, 108, 123] also reported an increased risk of rCDI, with aORs of 1.09 (95% CI 1.07–1.12) [107] and 3.55 (95% CI 1.41–8.94) [123].
Risk Factors for Mortality
Mortality after CDI diagnosis was described in 22 studies. Significantly increased risk of mortality with older age was reported in nine of 10 studies [25, 30, 124,125,126,127,128,129,130]. In the study by Appaneal et al., within a Veterans Affairs facility, age as a linear variable was associated with mortality: aOR per year increase 1.04 (95% CI 1.01–1.06; p value not presented) [124]. Results for sex as a risk factor for mortality were inconclusive. Two studies presented the OR for women versus men: one showed an increased risk (aOR 1.08, 95% CI 1.06–1.10; p value not presented) [125], whereas the other showed a decreased risk (aOR 0.78, 95% CI 0.60–0.99; p = 0.048) [25]. Another study presented the ORs for men versus women, reporting a significant increased risk in index encounters (aOR 1.17, 95% CI 1.05–1.31; p value not presented), but not in recurrent encounters (aOR 1.01, 95% CI 0.72–1.41). Two reports found that a body mass index (BMI) greater than 35–40 kg/m2 was associated with higher risk of in-hospital mortality (aOR 2.71, 95% CI 1.51–4.84) [25] and 30-day mortality (aOR 4.58, 95% CI 1.20–16.06) [129]; however, another reported no conclusive results related to a high BMI.
Four of five studies found that an ICU stay significantly increased the risk of mortality [121, 128, 129, 131]. In patients with cirrhosis and CDI, ICU was strongly associated with 30-day mortality (aOR 4.533, 95% CI 2.80–7.33; p < 0.0001) and overall mortality (aOR 2.232, 95% CI 1.32–3.79; p = 0.003) [131]. Antibiotic use was presented in three studies [124, 130, 131]. Any antibiotic use significantly increased the risk of overall mortality (aOR 3.33, 95% CI 1.79–6.17; p value not presented) [124]. Antibiotics during an initial CDI episode significantly increased the risk of 30-day mortality in patients with cirrhosis (aOR 2.186, 95% CI 1.23–3.89; p = 0.01) [131]. All-cause mortality was increased with extended-spectrum penicillin (aHR 1.86, 95% CI 1.1–3.2; p value not presented) but decreased with clindamycin (aHR 0.85, 95% CI 0.8–0.9; p value not presented) within 90 days of CDI diagnosis.
The effect of comorbidities on the risk of mortality was confounded by the limited number of reports and by conflicting results. Liver disease increased the risk of mortality or CDI-related mortality in three of five studies (aOR 1.65, 95% CI 1.53–1.77 [132]; aOR 2.00, 95% CI 1.78–2.25; aHR 3.31, 95% CI 1.19–9.25 [121]). Nutrition deficiency/malnutrition was a risk factor for all-cause mortality and CDI-related mortality (aOR 2.91, 95% CI 1.37–6.21 [124]; aOR 1.83, 95% CI 1.42–2.35) [79]. Increased risk of mortality was seen in two reports of autoimmune disease (aOR 1.39, 95% CI 0.73–2.65 [25]; and aOR 10.15, 95% CI 1.62–63.54 [127]) and one of two reports of infection (UTI aOR 1.36, 95% CI 1.35–1.37; sepsis aOR 3.54, 95% CI 3.47–3.60) [125]. In terms of bowel disease, ischemic bowel disease increased the risk of in-hospital death (aOR 4.94, 95% CI 3.91–6.41) [74] and inflammatory bowel disease (IBD) increased the risk of CDI-related mortality (aOR 1.72, 95% CI 1.49–1.99).
Time to CDI
The time to CDI was presented in 17 studies. Ten of the studies described CDI in a population of transplant recipients (stem cell transplant, n = 6; solid organ transplant, n = 4), five studies described CDI in patients undergoing surgery, and two studies were in other patient populations. In patients receiving stem cell transplant, the median time for CDI occurrence was generally in the first 2 or 3 weeks after transplantation [94, 105, 133,134,135,136]. In patients receiving organ transplantation, time to CDI varied but was often more than 3 weeks after transplantation [28, 41, 66]. In patients undergoing surgery, the mean or median times of CDI diagnoses were almost all within 3 weeks of the surgical procedure [61, 72, 76, 98, 137]. One study in patients admitted to ICU reported a median of 7 days to CDI onset [60]. Another study of patients with pancreatitis reported a mean time of 71 days following onset of necrotizing pancreatitis [71]. Three studies reported on the period between CDI and recurrence [41, 100, 138]: time varied widely between patients from a mean of 6.3 days in patients receiving high-risk antibiotics for an infection [100] to a median of 498 days in combined heart–lung transplant [138].
Discussion
This SLR identified likely risk factors for CDI as older age, female sex, the presence of certain comorbidities (including kidney disease, liver disease, cardiovascular disease, anemia, infections, and fluid and electrolyte disorders), use of antibiotics or PPIs, prior hospitalization, and a long LOS. Risk factors for rCDI were similar to those for initial CDI, including older age, use of antibiotics and PPI, and the presence of kidney disease, liver disease, and fluid and electrolyte disorders. Older age and an ICU stay were also identified as mortality risks. The time to occurrence of primary CDI was approximately 2–3 weeks in patients receiving stem cell transplantation, but longer for patients receiving solid organ transplantation.
Previous SLRs have confirmed that increasing age is a risk factor for CDI, rCDI, and mortality following CDI [21, 139, 140]. In our SLR, increasing age was a significant risk factor for primary CDI in almost all general population studies that reported on age. For specific populations, such as patients with IBD or cancer, age was not as common a risk factor, suggesting that other factors may be of greater importance in these populations. A previous report highlighted that, among an elderly population aged > 65 years, overall health status (including infection, acute conditions, comorbidities, frailty, and healthcare system utilization) was more important than age when predicting CDI risk [141]. Moreover, patients with CDI and IBD tend to be younger than those without IBD, likely as a result of the pathophysiology of IBD, which causes a disrupted gut microbial environment fundamental for C. difficile growth, thus increasing CDI risk regardless of age [142].
Increasing age was a potential risk factor for rCDI, although results were inconsistent. One study reported that younger patients (aged < 65 years) had a higher risk than those aged ≥ 65 years; however, younger patients were more likely to belong to the most disadvantaged neighborhoods, which had higher rates of comorbidities [108]. The lack of consistency with age as a risk factor for rCDI may also be due to the smaller pool size for patients with rCDI compared with primary CDI, making it more difficult for studies to detect significant differences. Increasing age was also a risk factor for mortality following CDI in almost all studies, although this result is confounded by the fact that the incidence of comorbidities increases with age [142].
Although an age cutoff of ≥ 65 years is often used in risk-estimate studies, particularly high-risk estimates for CDI at a cutoff of ≥ 50 years for infection and renal failure were found in the current SLR [56]. Future studies should investigate potentially increased risk beyond 50 years of age. Overall, older age is an important risk factor linked to immunosenescence, and is compounded by a greater exposure to the healthcare system, including antibiotics and hospitalization. CDI in younger patients may be linked to the presence of comorbidities, such as IBD or cancer.
In line with a previous report [21], we found female sex to be a risk factor for primary CDI. The reason for this is unknown, but female individuals are more likely to receive prescriptions for antibiotics than male individuals [143], which is linked to a higher tendency of women to seek medical care [144]. Therefore, greater exposure of female individuals to the healthcare system, including hospitalization, may explain the increased risk of primary CDI.
Comorbidities have long been considered potential risk factors for CDI [145]; however, our SLR suggests that some comorbidities are of greater importance than others. Kidney disease was a risk factor for primary CDI and rCDI, as has been previously reported [146]. Patients with chronic kidney disease are often old, with greater exposure to the healthcare system than the general population, and they typically have immune deficiencies with a reliance on antibiotics to fight infections, all of which contribute to an increased risk of CDI [146, 147]. Hypoalbuminemia and high serum creatinine levels are also associated with kidney disease and are further risk factors for CDI. Liver disease was a risk factor for CDI and rCDI; liver cirrhosis has previously been reported as a risk factor for CDI, potentially linked to immune dysfunction and hospitalization [148, 149]. Furthermore, CDI tends to be more severe in patients with cirrhosis, and has been linked to an increased risk of mortality [149]. Cardiovascular disease has previously been identified as a risk factor in primary CDI [21]. In particular, heart failure has been associated with an increased incidence of CDI and a risk of CDI-related mortality [150]. One potential reason for the increased risk of CDI may be reduced intestinal blood flow with heart failure, which could impact the intestinal microflora [151].
Our report also highlights several comorbidities not currently established as risk factors for CDI. For example, we identified 11 studies that reported anemia or low hematocrit as risk factors for CDI, and further investigation is warranted of anemia, coagulation disorders, fluid and electrolyte disorders, and being underweight as potential risk factors for CDI.
Four studies found that patients with versus those without obesity were less likely to experience CDI [34, 49, 52, 80]. In one of these studies, in CDI-associated pouchitis, a protective effect was seen at BMI > 25 kg/m2 [80]; another study, in surgical patients, found that the degree of protection increased with the level of obesity [52]. No clear effect of obesity was seen in seven other studies, including two studies in surgical patients with BMI cutoffs of > 30 kg/m2 [63] and ≥ 35 kg/m2 [53]. The reasons for the inter-study differences in effect are unclear.
Overweight (BMI ≥ 25 kg/m2) protected against CDI in other settings, including in elderly Japanese patients with pneumonia [152]. Despite this, we reported two of three studies where high BMI was a risk factor for mortality [25, 129]. More research is required to fully elucidate the impact of obesity and other factors (including BMI cutoffs) on the risk of developing CDI.
Diabetes, cancer, hypertension, and lung disease did not demonstrate a consistent, significantly increased risk of developing primary CDI, and further studies are required on these conditions. Although diabetes was reported as a risk factor for CDI in another SLR [21], our results were mixed and inconclusive. Patients with diabetes are at increased risk of infection and developing kidney disease [153], and may have high exposure to healthcare; these issues are potential risk factors for CDI. One potential reason for our findings could be the use of metformin in type 2 diabetes, which has been linked to protection from CDI [154]. The studies included in this review did not differentiate between type 1 or 2 diabetes; however, as type 2 diabetes accounts for 90–95% of diabetes cases in the USA [155], it is likely that most data included in the studies in this review were for type 2 diabetes. Although we examined only single comorbidities, the presence of multiple comorbidities could have had a cumulative effect. For example, another SLR reported that multiple comorbidities and increasing age (particularly > 65–70 years) were key risk factors for severe CDI [139]. In our review, risk factors for severe CDI were investigated, but were rarely reported in the constituent studies.
Antibiotic use is a risk factor for primary CDI and rCDI [21]. In line with previous findings, we found general antibiotic use to be a risk factor for primary CDI, and the risk was highest for cephalosporins, fluoroquinolones, lincosamides, and carbapenems. Three studies reported a protective effect for high-risk antibiotics: clindamycin in a general hospital population [88], fluoroquinolones in patients undergoing hematopoietic stem cell transplantation [94], and fluoroquinolones in a general hospital population [30]. The authors of these studies were unable to explain these surprising results, although Watson et al., proposed that their findings may have been due to a reduced use of clindamycin over time [88].
In this SLR, rCDI was associated with general antibiotic use before, during, or after primary CDI. A large-scale study showed that the use of fidaxomicin to treat primary CDI was associated with a reduced risk of recurrence compared with vancomycin [119]. This is in line with phase 3/4 studies and current treatment guidelines [156, 157]. Infection was also a risk factor for CDI, which is likely related to the routine use of antibiotics. In fact, there was considerable overlap in several risk factors. For example, the report of perineal or cesarean wound infections as significant risk factors for CDI could have been related to antibiotic treatment, being female, and hospitalization.
The time to CDI onset was largely studied in transplant recipients, although evidence was also available for patients undergoing surgery, patients attending an ICU, and patients with pancreatitis. A detailed comparison between patient groups was difficult because of the limited number of studies, small patient numbers, different follow-up periods, and differences in presentation of the results. CDI onset following stem cell transplantation is usually within the first month post-transplant [158], which is in line with our finding of 2–3 weeks. The incidence of CDI is generally two-fold higher in allogeneic compared with autologous transplants [158].
A recent database study of patients undergoing spinal surgery in Korea reported an average time of 18 days to CDI onset [159], which is in line with our findings for the time to CDI onset following surgery. A large Canadian study found that the risk of CDI differed with different solid organ transplants, with the incidence highest for multiorgan transplants (45.3 per 1000 person-years), followed by lung transplants (20.6 per 1000 person-years), and lowest for kidney allografts (9.6 per 1000 person-years) [160]. The researchers also reported that median time to CDI onset was longest for kidney transplants, at 2.2 years, and that late-onset CDI (≥ 90 days post-transplant) was associated with a greater than two-fold increased rate of mortality. Our SLR found that the time to CDI onset following solid organ transplantation was generally longer than that following stem cell transplantation. Together, these data highlight the need to consider long-term preventive measures in transplant recipients.
Only three studies investigated the time to CDI recurrence, two of which had mean or median values well beyond the generally accepted time of 2–8 weeks [11]. Furthermore, the definition of recurrence varied greatly across studies, highlighting the need to confirm a definition that can be used consistently in research and practice, whether the CDC definition or an adapted one.
Limitations
Limitations of this review include the heterogeneous nature of the constituent studies regarding designs, outcomes, patient populations, and definitions, making inter-study comparisons difficult. We searched two databases, which is regarded as the minimum required [161]. We considered that the two databases chosen (MEDLINE using PubMed, and EMBASE) would identify most of the relevant articles, especially regarding observational studies conducted in the USA. Reference lists from other SLRs were also checked for any articles that may have been missed (none were identified). We considered there was no additional benefit from using other databases; for example, CENTRAL contains mostly randomized controlled trials, which were not the focus of our search. Information on the definition or diagnosis of primary CDI was limited, so it was unclear if CDI was truly primary. Moreover, many studies were conducted retrospectively, making causality difficult to determine. CDI testing in most studies was conducted after symptoms occurred, and the status of “No CDI” was often not confirmed by testing. Furthermore, the recommended testing algorithms and the sensitivities of the tests may have changed over the past 5 years and could have been implemented to differing degrees across institutions and countries. Risk factors, such as comorbidities, were often grouped, although there was heterogeneity within the groups (i.e., conditions with varying causes that may have impacted differently on the risk of developing CDI). In the constituent studies of the review, dietary factors were generally not collected or reported; therefore, we could not generate a body of evidence robust enough to include in our analysis.
A meta-analysis was not conducted, as our fundamental research goal was to enhance understanding of the wider clinical context of CDI and not to specifically determine which comorbidities had the greatest impact in increasing CDI risk. As such, we agreed that a qualitative SLR would suffice. Moreover, given the marked heterogeneity of the studies and data (e.g., the different patient populations, subgroups, and CDI definitions), a meta-analysis would have provided limited additional information of clinical relevance.
Conclusion
In this SLR, evidence was strong for the established CDI risk factors of age and antibiotics, and certain comorbidities, including infection, kidney disease, liver disease, and cardiovascular disease. Several new risk factors for CDI were identified: anemia, underweight, coagulation disorders, and fluid and electrolyte disorders; these risk factors require further study to confirm the findings. Evidence was variable for cancer, diabetes, lung disease, and obesity, with further data required to fully understand the impact of these conditions on CDI. Risk factors for rCDI were similar to those for CDI, but evidence was more limited. In addition, age and antibiotics were important risk factors for mortality. Although data on the time to CDI onset were limited, strong evidence was provided for hematopoietic stem cell transplantation, in which CDI onset was generally within 3 weeks following transplantation; studies are needed to assess the time to CDI onset in other patient populations. Overall, this SLR provides recent evidence to define the most important risk factors associated with CDI, confirming those that are well established and highlighting new ones that could help to identify patient populations at high risk.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article. Most of the cited peer-reviewed publications of this study are available via PubMed, and the gray literature was derived from resources available in the public domain.
References
Czepiel J, Dróżdż M, Pituch H, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211–21. https://doi.org/10.1007/s10096-019-03539-6.
Schäffler H, Breitrück A. Clostridium difficile—from colonization to infection. Front Microbiol. 2018;9:646. https://doi.org/10.3389/fmicb.2018.00646.
CDC. Emerging infections program healthcare-associated infections—community interface report: Clostridioides difficile infection. 2019. https://www.cdc.gov/hai/eip/pdf/cdiff/2019-CDI-Report-H.pdf. Accessed Jun 2023.
Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. https://doi.org/10.1038/ajg.2013.4. (Quiz 99).
Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320–30. https://doi.org/10.1056/NEJMoa1910215.
Lessa FC, Winston LG, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–70. https://doi.org/10.1056/NEJMc1505190.
CDC. Emerging infections program healthcare-associated infections—community interface report. Clostridioides difficile infection. 2018. https://www.cdc.gov/hai/eip/Annual-CDI-Report-2018.html. Accessed Jun 2023.
Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–61. https://doi.org/10.1093/cid/cis462.
Reveles KR, Lawson KA, Mortensen EM, et al. National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014. PLoS ONE. 2017;12(12):e0189227. https://doi.org/10.1371/journal.pone.0189227.
Singh T, Bedi P, Bumrah K, Singh J, Rai M, Seelam S. Updates in treatment of recurrent Clostridium difficile infection. J Clin Med Res. 2019;11(7):465–71. https://doi.org/10.14740/jocmr3854.
CDC. Clostridioides difficile infection (CDI) tracking. https://www.cdc.gov/hai/eip/cdiff-tracking.html. Accessed Jun 2023.
Hopkins RJ, Wilson RB. Treatment of recurrent Clostridium difficile colitis: a narrative review. Gastroenterol Rep (Oxf). 2017;6(1):21–8. https://doi.org/10.1093/gastro/gox041.
Sartelli M, Di Bella S, McFarland LV, et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg. 2019;14:8. https://doi.org/10.1186/s13017-019-0228-3.
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. https://doi.org/10.1056/NEJMra1403772.
Abad CLR, Safdar N. A review of Clostridioides difficile infection and antibiotic-associated diarrhea. Gastroenterol Clin N Am. 2021;50(2):323–40. https://doi.org/10.1016/j.gtc.2021.02.010.
Falcone M, Tiseo G, Iraci F, et al. Risk factors for recurrence in patients with Clostridium difficile infection due to 027 and non-027 ribotypes. Clin Microbiol Infect. 2019;25(4):474–80. https://doi.org/10.1016/j.cmi.2018.06.020.
Goldenberg SD, Wigglesworth N, Wade P, Price NM. Effectiveness of early use of fidaxomicin in preventing recurrence of Clostridium difficile infection. J Hosp Infect. 2019;102(3):352–3. https://doi.org/10.1016/j.jhin.2019.01.013.
DuPont HL, Garey K, Caeiro JP, Jiang ZD. New advances in Clostridium difficile infection: changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis. 2008;21(5):500–7. https://doi.org/10.1097/QCO.0b013e32830f9397.
Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136(4):1206–14. https://doi.org/10.1053/j.gastro.2008.12.038.
Alrahmany D, Ereshefsky BJ, El Nekidy WS, Harb G, Pontiggia L, Ghazi IM. Risk factors for recurrence of Clostridioides difficile in hospitalized patients. J Infect Public Health. 2021;14(11):1642–9. https://doi.org/10.1016/j.jiph.2021.09.016.
Finn E, Andersson FL, Madin-Warburton M. Burden of Clostridioides difficile infection (CDI)—a systematic review of the epidemiology of primary and recurrent CDI. BMC Infect Dis. 2021;21(1):456. https://doi.org/10.1186/s12879-021-06147-y.
Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16–24. https://doi.org/10.5009/gnl18071.
Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Risk factors for recurrent Clostridium difficile infection (CDI) hospitalization among hospitalized patients with an initial CDI episode: a retrospective cohort study. BMC Infect Dis. 2014;14:306. https://doi.org/10.1186/1471-2334-14-306.
Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742–8. https://doi.org/10.1093/jac/dkr508.
Nathanson BH, Higgins TL, McGee WT. The dangers of extreme body mass index values in patients with Clostridium difficile. Infection. 2017;45(6):787–93. https://doi.org/10.1007/s15010-017-1036-x.
Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect (Larchmt). 2007;8(6):557–66. https://doi.org/10.1089/sur.2006.062.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, w64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
Jorgenson MR, Descourouez JL, Yang DY, et al. Epidemiology, risk factors, and outcomes after early posttransplant Clostridiodes difficile infection in renal transplant recipients. Ann Pharmacother. 2019;53(10):1020–5. https://doi.org/10.1177/1060028019845003.
Davis ML, Sparrow HG, Ikwuagwu JO, Musick WL, Garey KW, Perez KK. Multicentre derivation and validation of a simple predictive index for healthcare-associated Clostridium difficile infection. Clin Microbiol Infect. 2018;24(11):1190–4. https://doi.org/10.1016/j.cmi.2018.02.013.
Eberly MD, Susi A, Adams DJ, Love CS, Nylund CM. Epidemiology and outcomes of patients with healthcare facility-onset Clostridioides difficile infection. Mil Med. 2022;187(7–8):e915–20. https://doi.org/10.1093/milmed/usab116.
Harris AD, Sbarra AN, Leekha S, et al. Electronically available comorbid conditions for risk prediction of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol. 2018;39(3):297–301. https://doi.org/10.1017/ice.2018.10.
Lee DU, Fan GH, Ahern RR, Karagozian R. The effect of malnutrition on the infectious outcomes of hospitalized patients with cirrhosis: analysis of the 2011–2017 hospital data. Eur J Gastroenterol Hepatol. 2021;32(2):269–78. https://doi.org/10.1097/meg.0000000000001991.
Lorden AL, Jiang L, Radcliff TA, Kelly KA, Ohsfeldt RL. Potentially preventable hospitalizations and the burden of healthcare-associated infections. Health Serv Res Manag Epidemiol. 2017;4:2333392817721109. https://doi.org/10.1177/2333392817721109.
Miller AC, Sewell DK, Segre AM, Pemmaraju SV, Polgreen PM. Risk for Clostridioides difficile infection among hospitalized patients associated with multiple healthcare exposures prior to admission. J Infect Dis. 2021;224(4):684–94. https://doi.org/10.1093/infdis/jiaa773.
Press A, Ku B, McCullagh L, Rosen L, Richardson S, McGinn T. Developing a clinical prediction rule for first hospital-onset Clostridium difficile infections: a retrospective observational study. Infect Control Hosp Epidemiol. 2016;37(8):896–900. https://doi.org/10.1017/ice.2016.97.
Root ED, Lindstrom M, Xie A, Mangino JE, Moffatt-Bruce S, Hebert C. Investigating the association of room features with healthcare-facility-onset Clostridioides difficile: an exploratory study. Infect Control Hosp Epidemiol. 2021;42(7):847–52. https://doi.org/10.1017/ice.2020.1307.
Tilton CS, Johnson SW. Development of a risk prediction model for hospital-onset Clostridium difficile infection in patients receiving systemic antibiotics. Am J Infect Control. 2019;47(3):280–4. https://doi.org/10.1016/j.ajic.2018.08.021.
Webb BJ, Subramanian A, Lopansri B, et al. Antibiotic exposure and risk for hospital-associated Clostridioides difficile infection. Antimicrob Agents Chemother. 2020;64(4):e02169–19. https://doi.org/10.1128/aac.02169-19.
Keegan J, Buchan BW, Ledeboer NA, et al. Toxigenic Clostridioides difficile colonization as a risk factor for development of C. difficile infection in solid-organ transplant patients. Infect Control Hosp Epidemiol. 2020;42(3):287–91. https://doi.org/10.1017/ice.2020.431.
Rogala BG, Malat GE, Lee DH, Harhay MN, Doyle AM, Bias TE. Identification of risk factors associated with Clostridium difficile infection in liver transplantation recipients: a single-center analysis. Transplant Proc. 2016;48(8):2763–8. https://doi.org/10.1016/j.transproceed.2016.08.006.
Schluger A, Rosenblatt R, Knotts R, Verna EC, Pereira MR. Clostridioides difficile infection and recurrence among 2622 solid organ transplant recipients. Transpl Infect Dis. 2019;21(6):e13184. https://doi.org/10.1111/tid.13184.
Khazaaleh S, Gonzalez AJ, Alomari M, Wadhwa V, Shah B, Shen B. Ischemic colitis is a risk factor for Clostridium difficile infection. Cureus. 2022;14(6):e26076. https://doi.org/10.7759/cureus.26076.
Makar M, Makar G, Xia W, Greenberg P, Patel AV. Association of Clostridioides difficile with adverse clinical outcomes in patients with acute diverticulitis: a nationwide study. J Gastroenterol Hepatol. 2021;36(4):983–9. https://doi.org/10.1111/jgh.15240.
Palacios Argueta P, Salazar M, Attar B, Simons-Linares R, Shen B. 90-day specific readmission for Clostridium difficile infection after hospitalization with an inflammatory bowel disease flare: outcomes and risk factors. Inflamm Bowel Dis. 2021;27(4):530–7. https://doi.org/10.1093/ibd/izaa224.
Chang GY, Dembry LM, Banach DB. Epidemiology of Clostridium difficile infection in hospitalized oncology patients. Am J Infect Control. 2016;44(11):1408–10. https://doi.org/10.1016/j.ajic.2016.04.210.
Ford CD, Lopansri BK, Webb BJ, et al. Clostridioides difficile colonization and infection in patients with newly diagnosed acute leukemia: incidence, risk factors, and patient outcomes. Am J Infect Control. 2019;47(4):394–9. https://doi.org/10.1016/j.ajic.2018.09.027.
Kim D, Yoo ER, Li AA, Tighe SP, Cholankeril G, Ahmed A. Trends in hospitalizations for Clostridioides difficile infection in end-stage liver disease, 2005–2014. Dig Dis Sci. 2021;66(1):296–307. https://doi.org/10.1007/s10620-020-06162-0.
Ran-Castillo D, Oluwole A, Abuaisha M, et al. Risk, outcomes, and trends of Clostridium difficile infection in multiple myeloma patients from a nationwide analysis. Cureus. 2019;11(4):e4391. https://doi.org/10.7759/cureus.4391.
Adejumo AC, Adejumo KL, Pani LN. Risk and outcomes of Clostridium difficile infection with chronic pancreatitis. Pancreas. 2019;48(8):1041–9. https://doi.org/10.1097/mpa.0000000000001380.
Ge IY, Fevrier HB, Conell C, et al. Reducing risk of Clostridium difficile infection and overall use of antibiotic in the outpatient treatment of urinary tract infection. Ther Adv Urol. 2018;10(10):283–93. https://doi.org/10.1177/1756287218783871.
Kumar M, Peters M, Karabon P, Brahmamdam P. Clostridioides difficile infection after appendectomy: an analysis of short-term outcomes from the NSQIP database. Surgery. 2022;172(3):791–7. https://doi.org/10.1016/j.surg.2022.03.038.
Meier K, Nordestgaard AT, Eid AI, et al. Obesity as protective against, rather than a risk factor for, postoperative Clostridium difficile infection: a nationwide retrospective analysis of 1,426,807 surgical patients. J Trauma Acute Care Surg. 2019;86(6):1001–9. https://doi.org/10.1097/ta.0000000000002249.
Morales-Marroquin E, Xie L, Uppuluri M, Almandoz JP, Cruz-Muñoz N, Messiah SE. Immunosuppression and Clostridioides (Clostridium) difficile infection risk in metabolic and bariatric surgery patients. J Am Coll Surg. 2021;233(2):223–31. https://doi.org/10.1016/j.jamcollsurg.2021.04.028.
Nguyen KA, Le DQ, Bui YT, et al. Incidence, risk factors, and outcome of Clostridioides difficile infection following urological surgeries. World J Urol. 2021;39(8):2995–3003. https://doi.org/10.1007/s00345-020-03551-y.
Sahra S, Abureesh M, Amarnath S, et al. Clostridioides difficile infection in liver cirrhosis patients: a population-based study in United States. World J Hepatol. 2021;13(8):926–38. https://doi.org/10.4254/wjh.v13.i8.926.
Stevens VW, Russo EM, Young-Xu Y, et al. Identification of patients at risk of Clostridioides difficile infection for enrollment in vaccine clinical trials. Vaccine. 2021;39(3):536–44. https://doi.org/10.1016/j.vaccine.2020.12.016.
Belton PJ, Litofsky NS, Humphries WE. Effect of empiric treatment of asymptomatic bacteriuria in neurosurgical trauma patients on surgical site and Clostridium difficile infection. Neurosurgery. 2019;85(5):664–71. https://doi.org/10.1093/neuros/nyy430.
Gonzalez CA, Van RNL, Maschhoff C, Gardner MJ. Clostridium difficile colitis portends poor outcomes in lower extremity orthopaedic trauma surgery. Injury. 2022;53(10):3458–63. https://doi.org/10.1016/j.injury.2022.08.026.
Wang Y, Li J, Zachariah P, Abrams J, Freedberg DE. Relationship between remote cholecystectomy and incident Clostridioides difficile infection. Clin Microbiol Infect. 2019;25(8):994–9. https://doi.org/10.1016/j.cmi.2018.12.016.
Faleck DM, Salmasian H, Furuya EY, Larson EL, Abrams JA, Freedberg DE. Proton pump inhibitors do not increase risk for Clostridium difficile infection in the intensive care unit. Am J Gastroenterol. 2016;111(11):1641–8. https://doi.org/10.1038/ajg.2016.343.
Kirkwood KA, Gulack BC, Iribarne A, et al. A multi-institutional cohort study confirming the risks of Clostridium difficile infection associated with prolonged antibiotic prophylaxis. J Thorac Cardiovasc Surg. 2018;155(2):670-8.e1. https://doi.org/10.1016/j.jtcvs.2017.09.089.
Skancke M, Vaziri K, Umapathi B, Amdur R, Radomski M, Obias V. Elective stoma reversal has a higher incidence of postoperative Clostridium difficile infection compared with elective colectomy: an analysis using the American College of Surgeons National Surgical Quality Improvement Program and targeted colectomy databases. Dis Colon Rectum. 2018;61(5):593–8. https://doi.org/10.1097/dcr.0000000000001041.
Bell J, Vatani J, Raad M, Labaran L, Puvanesarajah V, Hassanzadeh H. Clostridium difficile infection following spine surgery: incidence, risk factors, and association with preoperative antibiotic use. Spine. 2020;45(22):1572–9. https://doi.org/10.1097/brs.0000000000003627.
Dubberke ER, Olsen MA, Stwalley D, et al. Identification of Medicare recipients at highest risk for Clostridium difficile infection in the US by population attributable risk analysis. PLoS ONE. 2016;11(2):e0146822. https://doi.org/10.1371/journal.pone.0146822.
Mamic P, Heidenreich PA, Hedlin H, Tennakoon L, Staudenmayer KL. Hospitalized patients with heart failure and common bacterial infections: a nationwide analysis of concomitant Clostridium difficile infection rates and in-hospital mortality. J Card Fail. 2016;22(11):891–900. https://doi.org/10.1016/j.cardfail.2016.06.005.
Sullivan T, Weinberg A, Rana M, Patel G, Huprikar S. The epidemiology and clinical features of Clostridium difficile infection in liver transplant recipients. Transplantation. 2016;100(9):1939–43. https://doi.org/10.1097/tp.0000000000001309.
Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Paren Enteral Nutr. 2019;43(2):181–93. https://doi.org/10.1002/jpen.1451.
Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, part III: albumin and disease states. JPEN J Paren Enteral Nutr. 1991;15(4):476–83.
Guh AY, Adkins SH, Li Q, et al. Risk factors for community-associated Clostridium difficile infection in adults: a case-control study. Open Forum Infect Dis. 2017;4(4):ofx171. https://doi.org/10.1093/ofid/ofx171.
Guzman JZ, Skovrlj B, Rothenberg ES, et al. The burden of Clostridium difficile after cervical spine surgery. Glob Spine J. 2016;6(4):314–21. https://doi.org/10.1055/s-0035-1562933.
Maatman TK, Westfall-Snyder JA, Nicolas ME, et al. The morbidity of C. difficile in necrotizing pancreatitis. Am J Surg. 2020;219(3):509–12. https://doi.org/10.1016/j.amjsurg.2019.08.006.
Cotter KJ, Fan Y, Sieger GK, Weight CJ, Konety BR. Prevalence of Clostridium difficile infection in patients after radical cystectomy and neoadjuvant chemotherapy. Bladder Cancer. 2017;3(4):305–10. https://doi.org/10.3233/blc-170132.
Bovonratwet P, Bohl DD, Malpani R, Nam D, Della Valle CJ, Grauer JN. Incidence, risk factors, and impact of Clostridium difficile colitis following primary total hip and knee arthroplasty. J Arthroplast. 2018;33(1):205-10.e1. https://doi.org/10.1016/j.arth.2017.08.004.
Adejumo AC, Akanbi O, Pani L. Among inpatients, ischemic bowel disease predisposes to Clostridium difficile infection with concomitant higher mortality and worse outcomes. Eur J Gastroenterol Hepatol. 2019;31(1):109–15. https://doi.org/10.1097/meg.0000000000001273.
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387–91. https://doi.org/10.1097/aog.0000000000002422.
Lynch KT, Cramer CL, Kane WJ, et al. A history of Clostridioides difficile infection portends infection recurrence and worse outcomes after stoma reversal. Surgery. 2021;170(1):55–60. https://doi.org/10.1016/j.surg.2020.12.032.
Elliott B, Czuzoj-Shulman N, Spence AR, Mishkin DS, Abenhaim HA. Effect of celiac disease on maternal and neonatal outcomes of pregnancy. J Matern Fetal Neonatal Med. 2021;34(13):2117–23. https://doi.org/10.1080/14767058.2019.1658733.
Chandradas S, Khalili H, Ananthakrishnan A, et al. Does obesity influence the risk of Clostridium difficile infection among patients with ulcerative colitis? Dig Dis Sci. 2018;63(9):2445–50. https://doi.org/10.1007/s10620-018-5108-2.
Lee DU, Fan GH, Hastie DJ, et al. The impact of malnutrition on the hospital and infectious outcomes of patients admitted with alcoholic hepatitis: 2011 to 2017 analysis of US hospitals. J Clin Gastroenterol. 2022;56(4):349–59. https://doi.org/10.1097/mcg.0000000000001528.
Gosai F, Covut F, Alomari M, et al. Obesity is associated with decreased risk of Clostridium difficile infection in hospitalized patients with pouchitis. Dig Dis Sci. 2020;65(5):1423–8. https://doi.org/10.1007/s10620-019-05888-w.
Voth E, Solanky D, Loftus EV Jr, Pardi DS, Khanna S. Novel risk factors and outcomes in inflammatory bowel disease patients with Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14:1756284821997792. https://doi.org/10.1177/1756284821997792.
Kamboj M, Gennarelli RL, Brite J, Sepkowitz K, Lipitz-Snyderman A. Risk for Clostridiodes difficile infection among older adults with cancer. Emerg Infect Dis. 2019;25(9):1683–9. https://doi.org/10.3201/eid2509.181142.
Suarez L, Kim J, Freedberg DE, Lebwohl B. Risk of healthcare-associated Clostridioides difficile infection during pandemic preparation: a retrospective cohort study. Gastro Hep Adv. 2022;1(1):8–11. https://doi.org/10.1016/j.gastha.2021.08.005.
Tilton CS, Sexton ME, Johnson SW, et al. Evaluation of a risk assessment model to predict infection with healthcare facility-onset Clostridioides difficile. Am J Health Syst Pharm. 2021;78(18):1681–90. https://doi.org/10.1093/ajhp/zxab201.
Parthasarathy M, Bowers D, Groot-Wassink T. Do preoperative oral antibiotics increase Clostridium difficile infection rates? An analysis of 13 959 colectomy patients. Colorectal Dis. 2018;20(6):520–8. https://doi.org/10.1111/codi.13926.
Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326–32.
Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951–61.
Watson T, Hickok J, Fraker S, Korwek K, Poland RE, Septimus E. Evaluating the risk factors for hospital-onset Clostridium difficile infections in a large healthcare system. Clin Infect Dis. 2018;66(12):1957–9. https://doi.org/10.1093/cid/cix1112.
Butler AM, Durkin MJ, Keller MR, Ma Y, Powderly WG, Olsen MA. Association of adverse events with antibiotic treatment for urinary tract infection. Clin Infect Dis. 2022;74(8):1408–18. https://doi.org/10.1093/cid/ciab637.
Song J, Cohen B, Liu J, Larson E, Zachariah P. The association between the frequency of interruptions in antibiotic exposure and the risk of health care-associated Clostridiodes difficile infection. Curr Ther Res Clin Exp. 2020;93:100600. https://doi.org/10.1016/j.curtheres.2020.100600.
Evans CT, Fitzpatrick M, Ramanathan S, et al. Healthcare facility-onset, healthcare facility-associated Clostridioides difficile infection in veterans with spinal cord injury and disorder. J Spinal Cord Med. 2020;43(5):642–52. https://doi.org/10.1080/10790268.2019.1672953.
Ely S, Rothenberg KA, Beattie G, Gologorsky RC, Huyser MR, Chang CK. Modern elective laparoscopic cholecystectomy carries extremely low postoperative infection risk. J Surg Res. 2020;246:506–11. https://doi.org/10.1016/j.jss.2019.09.038.
Carlson TJ, Gonzales-Luna AJ, Wilcox MF, et al. Corticosteroids do not increase the likelihood of primary Clostridioides difficile infection in the setting of broad-spectrum antibiotic use. Open Forum Infect Dis. 2021;8(10):ofab419. https://doi.org/10.1093/ofid/ofab419.
Aldrete SDM, Kraft CS, Magee MJ, et al. Risk factors and epidemiology of Clostridium difficile infection in hematopoietic stem cell transplant recipients during the peritransplant period. Transpl Infect Dis. 2017;19(1). https://doi.org/10.1111/tid.12649.
Karamanos E, Wang H, Shah AR. Clostridium difficile infection in the plastic surgery population: lessons from the ACS NSQIP database. Plast Reconstr Surg Glob Open. 2020;8(12):e3281. https://doi.org/10.1097/gox.0000000000003281.
Imlay H, Kaul D, Rao K. Risk factors for Clostridium difficile infection in HIV-infected patients. SAGE Open Med. 2016;4:2050312116684295. https://doi.org/10.1177/2050312116684295.
Sood G, Truelove S, Dougherty G, et al. Clostridioides difficile infection (CDI) in a previous room occupant predicts CDI in subsequent room occupants across different hospital settings. Am J Infect Control. 2022;50(12):1352–54. https://doi.org/10.1016/j.ajic.2022.02.006.
Coleoglou Centeno AA, Horn CB, Rasane RK, et al. Early emergency general surgery is associated with a higher incidence of Clostridium difficile infection. Surg Infect (Larchmt). 2019;20(1):10–5. https://doi.org/10.1089/sur.2018.183.
Wijarnpreecha K, Aby ES, Kim D, et al. The burden of Clostridioides difficile infection in patients with history of liver transplant and during index admission. Eur J Gastroenterol Hepatol. 2021;33(6):894–8. https://doi.org/10.1097/meg.0000000000001812.
Wombwell E. Saccharomyces boulardii prophylaxis for targeted antibiotics and infectious indications to reduce healthcare facility-onset Clostridioides difficile infection. Microbes Infect. 2023;25(3):105041. https://doi.org/10.1016/j.micinf.2022.105041.
Chauv S, Fontaine GV, Hoang QP, et al. Risk of resistant organisms and Clostridium difficile with prolonged systemic antibiotic prophylaxis for central nervous system devices. Neurocrit Care. 2016;25(1):128–32. https://doi.org/10.1007/s12028-016-0254-x.
Shah PJ, Halawi H, Kay J, et al. A single-center, retrospective cohort study evaluating the use of probiotics for the prevention of hospital-onset Clostridioides difficile infection in hospitalized patients receiving intravenous antibiotics. Hosp Pharm. 2023;58(1):57–61. https://doi.org/10.1177/00185787221120153.
Carvour ML, Wilder SL, Ryan KL, et al. Predictors of Clostridium difficile infection and predictive impact of probiotic use in a diverse hospital-wide cohort. Am J Infect Control. 2019;47(1):2–8. https://doi.org/10.1016/j.ajic.2018.07.014.
Bovonratwet P, Bohl DD, Russo GS, et al. How common—and how serious—is Clostridium difficile colitis after geriatric hip fracture? Findings from the NSQIP dataset. Clin Orthop Relat Res. 2018;476(3):453–62. https://doi.org/10.1007/s11999.0000000000000099.
Jain T, Croswell C, Urday-Cornejo V, et al. Clostridium difficile colonization in hematopoietic stem cell transplant recipients: a prospective study of the epidemiology and outcomes involving toxigenic and nontoxigenic strains. Biol Blood Marrow Transplant. 2016;22(1):157–63. https://doi.org/10.1016/j.bbmt.2015.07.020.
Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med. 2017;167(3):152–8. https://doi.org/10.7326/m16-2733.
Mathews SN, Lamm R, Yang J, et al. Factors associated with health care utilization of recurrent Clostridium difficile infection in New York state. J Clin Gastroenterol. 2019;53(4):298–303. https://doi.org/10.1097/mcg.0000000000001022.
Scaria E, Powell WR, Birstler J, et al. Neighborhood disadvantage and 30-day readmission risk following Clostridioides difficile infection hospitalization. BMC Infect Dis. 2020;20(1):762. https://doi.org/10.1186/s12879-020-05481-x.
Mani S, Rybicki L, Jagadeesh D, Mossad SB. Risk factors for recurrent Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Bone Marrow Transplant. 2016;51(5):713–7. https://doi.org/10.1038/bmt.2015.311.
Rao K, Higgins PDR, Young VB. An observational cohort study of Clostridium difficile ribotype 027 and recurrent infection. mSphere. 2018;3(3):e00033–18. https://doi.org/10.1128/mSphere.00033-18.
Sutton SS, Magagnoli J, Cummings TH, Hardin JW. Melatonin as an antimicrobial adjuvant and anti-inflammatory for the management of recurrent Clostridioides difficile infection. Antibiotics (Basel). 2022;11(11):1472. https://doi.org/10.3390/antibiotics11111472.
Benitez G, Shehadeh F, Kalligeros M, et al. Risk factors for hospital readmission for Clostridioides difficile infection: a statewide retrospective cohort study. Pathogens. 2022;11(5):555. https://doi.org/10.3390/pathogens11050555.
Douglas SJ, Remily EA, Sax OC, Pervaiz SS, Polsky EB, Delanois RE. THAs performed within 6 months of Clostridioides difficile infection are associated with increased risk of 90-day complications. Clin Orthop Relat Res. 2021;479(12):2704–11. https://doi.org/10.1097/corr.0000000000001837.
El HJ, Palmer N, Fox K, Kohane I, Farhat MR. Fecal microbiota transplantation and Clostridioides difficile infection among privately insured patients in the United States. J Gastroenterol. 2022;57(1):10–8. https://doi.org/10.1007/s00535-021-01822-y.
Verheyen E, Dalapathi V, Arora S, et al. High 30-day readmission rates associated with Clostridium difficile infection. Am J Infect Control. 2019;47(8):922–7. https://doi.org/10.1016/j.ajic.2019.01.007.
Young MK, Leslie JL, Madden GR, et al. Binary toxin expression by Clostridioides difficile is associated with worse disease. Open Forum Infect Dis. 2022;9(3):ofac01. https://doi.org/10.1093/ofid/ofac001.
Appaneal HJ, Caffrey AR, Beganovic M, Avramovic S, LaPlante KL. Predictors of Clostridioides difficile recurrence across a national cohort of veterans in outpatient, acute, and long-term care settings. Am J Health Syst Pharm. 2019;76(9):581–90. https://doi.org/10.1093/ajhp/zxz032.
Scappaticci GB, Perissinotti AJ, Nagel JL, Bixby DL, Marini BL. Risk factors and impact of Clostridium difficile recurrence on haematology patients. J Antimicrob Chemother. 2017;72(5):1488–95. https://doi.org/10.1093/jac/dkx005.
Hall RG 2nd, Cole TJ, Shaw C, Alvarez CA. The risk of Clostridioides difficile recurrence after initial treatment with vancomycin or fidaxomicin utilizing cerner health facts. Antibiotics (Basel). 2022;11(3):295. https://doi.org/10.3390/antibiotics11030295.
Haran JP, Bradley E, Howe E, Wu X, Tjia J. Medication exposure and risk of recurrent Clostridium difficile infection in community-dwelling older people and nursing home residents. J Am Geriatr Soc. 2018;66(2):333–8. https://doi.org/10.1111/jgs.15176.
Phatharacharukul P, Purpura RD, Gandhi D, et al. Incidence and risk factors of recurrent Clostridioides difficile infection in patients with cirrhosis. Clin Transl Gastroenterol. 2020;11(7):e00189. https://doi.org/10.14309/ctg.0000000000000189.
Weber BR, Noble BN, Bearden DT, et al. Antibiotic prescribing upon discharge from the hospital to long-term care facilities: implications for antimicrobial stewardship requirements in post-acute settings. Infect Control Hosp Epidemiol. 2018;40(1):18–23. https://doi.org/10.1017/ice.2018.288.
Jasiak NM, Alaniz C, Rao K, Veltman K, Nagel JL. Recurrent Clostridium difficile infection in intensive care unit patients. Am J Infect Control. 2016;44(1):36–40. https://doi.org/10.1016/j.ajic.2015.08.013.
Appaneal HJ, Caffrey AR, Beganovic M, Avramovic S, LaPlante KL. Predictors of mortality among a national cohort of veterans with recurrent Clostridium difficile infection. Open Forum Infect Dis. 2018;5(8):ofy175. https://doi.org/10.1093/ofid/ofy175.
Argamany JR, Delgado A, Reveles KR. Clostridium difficile infection health disparities by race among hospitalized adults in the United States, 2001 to 2010. BMC Infect Dis. 2016;16(1):454. https://doi.org/10.1186/s12879-016-1788-4.
Bradley ES, Howe E, Wu X, Haran JP. Proton pump inhibitors and 180-day mortality in the elderly after Clostridium difficile treatment. Gut Pathog. 2019;11:29. https://doi.org/10.1186/s13099-019-0309-6.
Figh ML, Zoog ESL, Moore RA, et al. External validation of Velazquez-Gomez severity score index and ATLAS scores and the identification of risk factors associated with mortality in Clostridium difficile infections. Am Surg. 2017;83(12):1347–51.
Kassam Z, Cribb FC, Smith MB, et al. Clostridium difficile associated risk of death score (CARDS): a novel severity score to predict mortality among hospitalised patients with C. difficile infection. Aliment Pharmacol Ther. 2016;43(6):725–33. https://doi.org/10.1111/apt.13546.
Malick A, Wang Y, Axelrad J, Salmasian H, Freedberg D. Obesity is not associated with adverse outcomes among hospitalized patients with Clostridioides difficile infection. Gut Pathog. 2022;14(1):7. https://doi.org/10.1186/s13099-022-00479-z.
Mora PMC, Buie R, Liou JI, et al. Outcomes of community and healthcare-onset Clostridium difficile infections. Clin Infect Dis. 2019;68(8):1343–50. https://doi.org/10.1093/cid/ciy715.
Smith EZ, Northup PG, Argo CK. Predictors of mortality in cirrhosis inpatients with Clostridium difficile infection. J Clin Gastroenterol. 2018;52(8):747–51. https://doi.org/10.1097/MCG.0000000000000868.
Abdalla AO, Pisipati S, Elnaggar M, Rishi M, Doshi R, Gullapalli N. Outcomes of Clostridioides difficile infection in patients with liver cirrhosis: a nationwide study. Gastroenterol Res. 2020;13(2):53–7. https://doi.org/10.14740/gr1240.
Alonso CD, Braun DA, Patel I, et al. A multicenter, retrospective, case-cohort study of the epidemiology and risk factors for Clostridium difficile infection among cord blood transplant recipients. Transpl Infect Dis. 2017;19(4). https://doi.org/10.1111/tid.12728.
Dubberke ER, Reske KA, Olsen MA, et al. Risk for Clostridium difficile infection after allogeneic hematopoietic cell transplant remains elevated in the postengraftment period. Transplant Direct. 2017;3(4):e145. https://doi.org/10.1097/txd.0000000000000662.
Agha A, Sehgal A, Lim MJ, et al. Peri-transplant Clostridium difficile infections in patients undergoing allogeneic hematopoietic progenitor cell transplant. Am J Hematol. 2016;91(3):291–4. https://doi.org/10.1002/ajh.24263.
Scardina TL, Kang Martinez E, Balasubramanian N, Fox-Geiman M, Smith SE, Parada JP. Evaluation of risk factors for Clostridium difficile infection in hematopoietic stem cell transplant recipients. Pharmacotherapy. 2017;37(4):420–8. https://doi.org/10.1002/phar.1914.
Kochhar G, Edge P, Blomme C, et al. Clostridium difficle enteropathy is associated with a higher risk for acute kidney injury in patients with an ileostomy—a case–control study. Inflamm Bowel Dis. 2018;24(2):402–9. https://doi.org/10.1093/ibd/izx034.
Bruminhent J, Cawcutt KA, Thongprayoon C, Petterson TM, Kremers WK, Razonable RR. Epidemiology, risk factors, and outcome of Clostridium difficile infection in heart and heart-lung transplant recipients. Clin Transplant. 2017;31(6). https://doi.org/10.1111/ctr.12968.
van Rossen TM, Ooijevaar RE, Vandenbroucke-Grauls CMJE, et al. Prognostic factors for severe and recurrent Clostridioides difficile infection: a systematic review. Clin Microbiol Infect. 2022;28(3):321–31. https://doi.org/10.1016/j.cmi.2021.09.026.
Abou CCN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS ONE. 2014;9(6):e98400. https://doi.org/10.1371/journal.pone.0098400.
Olsen MA, Stwalley D, Demont C, Dubberke ER. Increasing age has limited impact on risk of Clostridium difficile infection in an elderly population. Open Forum Infect Dis. 2018;5(7):ofy160. https://doi.org/10.1093/ofid/ofy160.
Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(4):1055–68. https://doi.org/10.1183/09031936.00059814.
Schröder W, Sommer H, Gladstone BP, et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1800–6. https://doi.org/10.1093/jac/dkw054.
Pinkhasov RM, Wong J, Kashanian J, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract. 2010;64(4):475–87. https://doi.org/10.1111/j.1742-1241.2009.02290.x.
Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(2):132–41. https://doi.org/10.1017/ice.2014.39.
Phatharacharukul P, Thongprayoon C, Cheungpasitporn W, Edmonds PJ, Mahaparn P, Bruminhent J. The risks of incident and recurrent Clostridium difficile-associated diarrhea in chronic kidney disease and end-stage kidney disease patients: a systematic review and meta-analysis. Dig Dis Sci. 2015;60(10):2913–22. https://doi.org/10.1007/s10620-015-3714-9.
Dudzicz S, Wiecek A, Adamczak M. Clostridioides difficile infection in chronic kidney disease—an overview for clinicians. J Clin Med. 2021;10(2):196. https://doi.org/10.3390/jcm10020196.
Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19(2):112–34. https://doi.org/10.1038/s41575-021-00520-7.
Mantri N, Patel H, Badipatla KR, et al. Clostridioides difficile infection and liver cirrhosis—a retrospective, cohort study. Clin Exp Gastroenterol. 2021;14:229–35. https://doi.org/10.2147/ceg.S308862.
Méndez-Bailón M, Jiménez-García R, Hernández-Barrera V, et al. Heart failure is a risk factor for suffering and dying of Clostridium difficile infection. Results of a 15-year nationwide study in Spain. J Clin Med. 2020;9(3):614. https://doi.org/10.3390/jcm9030614.
Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64(11):1092–102. https://doi.org/10.1016/j.jacc.2014.06.1179.
Suzuki R, Sakata N, Fushimi K. Association of body mass index with Clostridioides difficile infection among older patients with pneumonia in Japan. Geriatr Gerontol Int. 2022;22(1):63–7. https://doi.org/10.1111/ggi.14316.
Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442–9. https://doi.org/10.2174/1573399815666191024085838.
Eliakim-Raz N, Fishman G, Yahav D, et al. Predicting Clostridium difficile infection in diabetic patients and the effect of metformin therapy: a retrospective, case-control study. Eur J Clin Microbiol Infect Dis. 2015;34(6):1201–5. https://doi.org/10.1007/s10096-015-2348-3.
CDC. Diabetes. 2023. https://www.cdc.gov/nchs/fastats/diabetes.htm. Accessed 11 Aug 2023.
Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–44. https://doi.org/10.1093/cid/ciab549.
Giacobbe DR, Vena A, Falcone M, Menichetti F, Bassetti M. Fidaxomicin for the treatment of Clostridioides difficile infection in adult patients: an update on results from randomized controlled trials. Antibiotics (Basel). 2022;11(10):1365. https://doi.org/10.3390/antibiotics11101365.
Misch EA, Safdar N. Clostridioides difficile infection in the stem cell transplant and hematologic malignancy population. Infect Dis Clin North Am. 2019;33(2):447–66. https://doi.org/10.1016/j.idc.2019.02.010.
Sung S, Kwon J-W, Lee S-B, Lee H-M, Moon S-H, Lee BH. Risk factors of Clostridium difficile infection after spinal surgery: national health insurance database. Sci Rep. 2020;10(1):4438. https://doi.org/10.1038/s41598-020-61327-1.
Hosseini-Moghaddam SM, Luo B, Bota SE, et al. Incidence and outcomes associated with Clostridioides difficile infection in solid organ transplant recipients. JAMA Netw Open. 2021;4(12):e2141089. https://doi.org/10.1001/jamanetworkopen.2021.41089.
Ewald H, Klerings I, Wagner G, et al. Searching two or more databases decreased the risk of missing relevant studies: a metaresearch study. J Clin Epidemiol. 2022;149:154–64. https://doi.org/10.1016/j.jclinepi.2022.05.022.
CDC. Pneumonia. 2021. https://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 26 Jul 2021.
CDC. Kidney disease. 2021. https://www.cdc.gov/nchs/fastats/kidney-disease.htm. Accessed 26 Jul 2021.
CDC. Chronic liver disease and cirrhosis. 2021. https://www.cdc.gov/nchs/fastats/liver-disease.htm. Accessed 26 Jul 2021.
CDC. Heart disease. 2021. https://www.cdc.gov/nchs/fastats/heart-disease.htm. Accessed 26 Jul 2021.
Alonso A, Aparicio FHJ, Benjamin EJ, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;2021(143):e00-e.
Le CHH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012). PLoS ONE. 2016;11(11):e0166635.
CDC. Inflammatory bowel disease (IBD) in the United States. 2021. https://www.cdc.gov/ibd/data-statistics.htm#:~:text=Inflammatory%20Bowel%20Disease%20Prevalence%20(IBD,%25%20or%202%20million%20adults). Accessed 26 Jul 2021.
CDC. Data & statistics on hemophilia. 2020. https://www.cdc.gov/ncbddd/hemophilia/data.html. Accessed 26 Jul 2021.
National Bleeding Disorders Foundation. 2021. https://www.hemophilia.org/bleeding-disorders-a-z/overview/fast-facts. Accessed 26 Jul 2021.
Cheryl D. Fryar MDC, Cynthia L. Ogden. Prevalence of underweight among adults aged 20 and over: United States, 1960–1962 through 2015–2016. 2018. https://www.cdc.gov/nchs/data/hestat/underweight_adult_15_16/underweight_adult_15_16.htm. Accessed 26 Jul 2021.
Boscoe A, Paramore C, Verbalis JG. Cost of illness of hyponatremia in the United States. Cost Effect Resour Alloc. 2006;4(1):1–11.
CDC. Cancer. 2021. https://www.cdc.gov/nchs/fastats/cancer.htm. Accessed 26 Jul 2021.
CDC. Asthma. 2021. https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed 26 Jul 2021.
CDC. Chronic obstructive pulmonary disease (COPD) includes: chronic bronchitis and emphysema. 2021. https://www.cdc.gov/nchs/fastats/copd.htm. Accessed 26 Jul 2021.
CDC. Facts about hypertension. 2021. https://www.cdc.gov/bloodpressure/facts.htm. Accessed 26 Jul 2021.
CDC. Adult obesity facts. 2021. https://www.cdc.gov/obesity/data/adult.html. Accessed 26 Jul 2021
Acknowledgements
Medical Writing and Editorial Assistance.
Medical writing support was provided by David Murdoch, a contract writer working on behalf of Apollo, and Lewis Cawkwell, of Apollo, OPEN Health Communications, funded by GSK, in accordance with Good Publication Practice 3 GPP) guidelines (www.ismpp.org/gpp-2022). Eveline Bunge of Pallas Health Research and Consultancy, Ornella Ruiz and Egbe Sheila Ubamadu of P95 were involved in the selection, reviewing, and extraction for the systematic literature review.
Funding
This article was supported by GSK Biologicals SA. GSK Biologicals SA also took in charge all costs associated with the development and publication of this manuscript, including the journal’s Rapid Service fee.
Author information
Authors and Affiliations
Contributions
All authors were involved in the planning, discussion, and interpretation of the data. All reviewed and revised the manuscript and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of Interest
Gui Ferreira and Juan Pablo Yarzabal are employees of GSK, and hold or may hold stock options. Jennifer Eeuwijk is an employee of Pallas Health Research and Consultancy. Mirna Robert-Du Ry van Beest Holle is owner of Mirna Robert Epidemiology d.o.o., and she was a freelance consultant for GSK until June 2023. Mirna Robert-Du Ry van Beest Holle holds no GSK stock options.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eeuwijk, J., Ferreira, G., Yarzabal, J.P. et al. A Systematic Literature Review on Risk Factors for and Timing of Clostridioides difficile Infection in the United States. Infect Dis Ther 13, 273–298 (2024). https://doi.org/10.1007/s40121-024-00919-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00919-0