Abstract
Introduction
US pneumococcal vaccination recommendations for adults aged 65 years or older recently changed, with options for either 20-valent pneumococcal conjugate vaccine (PCV20) or the combination of 15-valent conjugate vaccine (PCV15) followed by 23-valent polysaccharide vaccine (PPSV23) 1 year later. Underserved minority adults are at higher risk for pneumococcal disease.
Methods
A Markov decision analysis model estimated the incremental cost-effectiveness of the newly adopted general population pneumococcal vaccination strategies in older underserved minority adults. The model examined hypothetical 65-year-old US Black cohorts (serving as a proxy for underserved minorities) and non-Black cohorts receiving PCV20 or PCV15/PPSV23, or no vaccination. Main outcome measures included incremental cost-effectiveness per quality-adjusted life year (QALY) gained and pneumococcal disease public health outcomes.
Results
Black cohorts had a greater risk of pneumococcal disease hospitalization compared to non-Black cohorts. In Black cohorts, total per person PCV20 strategy costs, compared to no vaccination, were $124 higher while gaining 0.00073 QALY, or $169,540/QALY gained. PCV15/PPSV23 cost $535,797/QALY compared to PCV20. In the non-Black cohort, PCV20 cost $210,529/QALY gained compared to no vaccination and PCV15/PPSV23 cost $728,423/QALY. Plausible variation of vaccine effectiveness minimally affected PCV20 strategy results and made PCV15/PPSV23 more unfavorable. In scenarios where the simpler one-vaccine PCV20 strategy increased absolute vaccine uptake by 10%, PCV20 cost-effectiveness changed minimally while PCV15/PPSV23 cost in excess of $6 million/QALY in the Black cohort. In probabilistic sensitivity analyses that varied all parameters simultaneously, PCV15/PPSV23 was unlikely to be favored at thresholds less than $500,000/QALY gained.
Conclusion
General population recommendations for PCV20 use are substantially more economically reasonable in Black and non-Black older adult populations than PCV15/PPSV23. If using a single vaccine increases uptake, which is potentially more likely in the underserved, then PCV20 use becomes even more favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
US pneumococcal vaccination recommendations for adults aged 65 years and older recently changed, with options for either 20-valent pneumococcal conjugate vaccine (PCV20) or the combination of 15-valent conjugate vaccine (PCV15) followed by 23-valent polysaccharide vaccine (PPSV23). |
The cost-effectiveness of these recommended options in US underserved minority populations is unclear. |
What was learned from the study? |
In a decision analysis model, PCV20 use was substantially more economically favorable than PCV15/PPSV23 in both Black and non-Black cohorts, with results that were robust to variation in sensitivity analyses. |
If the single-vaccine PCV20 increases vaccine uptake, which is potentially more likely in the underserved, then PCV20 use becomes even more highly favorable for this group. |
PCV20 use in US underserved minority older adults could decrease pneumococcal disease burden and mitigate disease disparities in an economically reasonable fashion. |
Introduction
In 2021, two new higher valency conjugate pneumococcal vaccines were licensed for use in US adults. The Centers for Disease Control and Prevention (CDC) subsequently revised the US pneumococcal vaccination schedule for adults aged 65 years and older to include those vaccines, with options to use either 20-valent pneumococcal conjugate vaccine (PCV20) or the combination of 15-valent conjugate vaccine (PCV15) followed by 23-valent polysaccharide vaccine (PPSV23) 1 year later [1]. When compared to prior recommendations, CDC expects that these newly recommended strategies will reduce adult pneumococcal disease incidence and simplify adult pneumococcal vaccination [1]. Compared to the 13-valent pneumococcal conjugate vaccine (PCV13), PCV15 adds coverage for serotypes 22F and 33F while PCV20 adds serotypes 8, 10A, 11A, 12F, 15F, 22F, and 33F, increasing pneumococcal serotype coverage by an absolute 15–29%, respectively. [2] Additional considerations for these changes in recommendations came from CDC- and industry-sponsored cost-effectiveness analyses [2,3,4] comparing the clinical and economic favorability of these new strategies to prior recommendations under various model assumptions and data estimates. These analyses, for the most part, found that using either of the newer vaccines was economically favorable to the prior recommendation. However, based on guidance from the CDC’s Advisory Committee on Immunization Practices (ACIP), the two newer vaccine strategies were compared to the prior recommendation and not to each other [4].
Prior work has shown that Black populations, which can be used as a proxy for medically underserved populations in the USA, have greater pneumococcal disease risks and costs [5]. In addition, we previously demonstrated that policies or programs to increase general population pneumococcal vaccination rates can disproportionately affect the underserved and potentially reduce these disparities while remaining economically reasonable [6, 7]. Strategies to increase vaccine uptake reduce barriers to access by assessing need and offering vaccines at every healthcare system contact, offering simultaneous vaccinations, and removing out-of-pocket costs, among other interventions [8]. Many of these strategies assume that individuals have a primary care clinician or a medical home with regular visits, which is often not the case with medically underserved populations. Thus, a recommended vaccination strategy that requires two visits, such as the PCV15/PPSV23 strategy, where PPSV23 is given at least 1 year after PCV15, may result in lower vaccination series completion and greater pneumococcal disease risk for the medically underserved compared to a less complex strategy.
In this decision model-based cost-effectiveness analysis, we compare the newly recommended US pneumococcal vaccination options among medically underserved and non-underserved adults aged 65 years and older. In these analyses, we examine the potential effects of various assumptions regarding vaccine effectiveness and differential uptake between vaccination strategies, focusing on how those changes might affect the favorability of general population pneumococcal vaccination strategies in medically underserved minority older adults.
Methods
A Markov state transition model estimated the incremental cost-effectiveness of pneumococcal vaccination strategies in a hypothetical 1-year age cohort of US Black population 65-year-olds, serving as a proxy for the US medically underserved minority population. Three vaccination strategies, reflecting new US policy options and available vaccines, were examined: (1) PCV20, (2) PCV15 followed by PPSV23 1 year later, and (3) no pneumococcal vaccination. These strategies were also separately examined in a US 65-year-old non-Black cohort. Cohort sizes were based on US Census data on 65-year-olds in 2020 [9]. Analyses took a healthcare perspective, following cohorts yearly through a lifetime time horizon, with future costs and effectiveness discounted at 3% per year. Vaccine costs are in 2022 US dollars; all other costs are in 2017 US dollars, based on the epidemiologic data used, with prior costs inflated using the US Consumer Price Index.
This article is based on data from previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Cohorts were segmented on the basis of the presence or absence of chronic health conditions relevant to differential pneumococcal disease risk, using age- and race-specific 2013–2014 National Health Interview Survey and CDC data as previously described [5, 10]. Age- and race-specific all-cause mortality was obtained from National Center for Health Statistics (NCHS) life tables. CDC and US databases informed age- and race-specific epidemiologic and disease-related resource utilization parameters, as outlined below. Vaccine costs were obtained from the CDC vaccine price list [11].
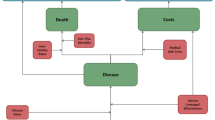
The Markov model is depicted schematically in Supplementary Fig. 1. Cohorts enter the model at age 65 years in one of four health states: average risk, average risk smoker, immunocompromising condition, or chronic medical conditions (CMC) with their attendant risks and vaccine effectiveness estimates. CMCs are non-immunocompromising conditions conferring high risk of pneumococcal disease. CDC definitions for CMC and immunocompromising conditions were used to segment cohorts [1]. Cohorts were vaccinated, based on age- and race-specific pneumococcal vaccination likelihood. Vaccinated or not, cohorts were subject to pneumococcal disease, with illness risk in the vaccinated equaling illness risk in the unvaccinated multiplied by 1 minus vaccine effectiveness values specific to age, health condition, and time since vaccination. Those who become ill with pneumococcal disease can die, recover completely, or become disabled as a result of illness. Disability risk was based on pneumococcal meningitis risk, understanding that all meningitis is not disabling and that other pneumococcal diseases can lead to disability. Pneumococcal illness, mortality, and disability risks in hospitalized patients with nonbacteremic pneumococcal pneumonia (NBP) were assumed to be 50% of that observed in invasive pneumococcal disease (IPD). In addition, with each yearly cycle of the model, individuals can develop CMC or immunocompromising conditions and transition to the appropriate health state and thus assume the pneumococcal disease risk and vaccine effectiveness associated with that state. The Markov model cycles until the entire cohort reaches 100 years of age, using age- and race-specific mortality risk as outlined above.
Model parameter values are listed in Supplementary Table 1. Age-, race-, and health state-specific IPD incidence data were derived from CDC 2017–2018 Active Bacterial Core surveillance (ABCs) data. IPD is defined as pneumococcal infection in an otherwise sterile environment, most commonly in the bloodstream. These data include the likelihood of IPD caused by serotypes contained in each of the vaccines.
Vaccine effectiveness values used in this analysis are depicted in Supplementary Table 2. Vaccine effectiveness in preventing disease from serotypes contained in the vaccines (except for serotype 3) were largely, but not wholly, derived from values used in CDC-sponsored, cost-effectiveness analyses [3]. Vaccine effectiveness values used in our analysis differed from CDC values in that, in our base case analysis, PPSV23 had greater effectiveness in preventing IPD. Vaccine effectiveness values that differ from those used in the CDC analysis were previously obtained from expert panels using the modified Delphi technique and are detailed in Supplementary Table 2. All vaccine effectiveness values were examined in our sensitivity analyses, with differing values and value sets used to test the robustness of analysis results. PCV13 has been reported to have decreased effectiveness against pneumococcal serotype 3 [12]. We assume that PCV15 and PCV20 have similarly reduced serotype 3 effectiveness but relax that assumption in sensitivity analyses allowing differential effectiveness among vaccine products. All other vaccine serotypes were assumed to be equally affected by their respective vaccines. CDC values for differential serotype 3 effectiveness were examined in sensitivity analyses [3]. Our modeling of PCV15/PPSV23 effectiveness assumes that all persons receiving PCV15 will also receive PPSV23 1 year later, potentially biasing results toward the PCV15/PPSV23 strategy.
Pneumococcal disease costs were also derived from CDC-sponsored analyses (Supplementary Table 1). In our analysis, we assume that illness costs do not differ based on patient health state, race, or discharge status. The CDC analysis assumed some differences in pneumococcal disease costs based on chronic health status [3].
Quality of life utilities for pneumococcal disease health states were obtained from the medical literature (Supplementary Table 1). Utilities range from 0 (death) to 1 (perfect health). Quality-adjusted life years (QALY), the effectiveness term in the analysis, are the product of health state utilities multiplied by time in health states summed over all health states over time.
Hospitalized NBP risk was estimated to be three times the observed rate of bacteremic pneumococcal pneumonia, a component of IPD, using the method of Said et al. [13] The distribution of pneumococcal serotypes causing hospitalized NBP was assumed to be identical to that seen in patients with IPD. Non-hospitalized pneumococcal pneumonia risk was derived as a proportion of all-cause pneumonia, as previously, and varied widely in sensitivity analyses given its uncertainty [7].
One-way sensitivity analyses were performed, individually varying all parameter values, to identify parameters whose variation might affect preference for a given strategy. In addition, probabilistic sensitivity analyses were performed, varying all parameter values simultaneously over distributions 3000 times. Cost parameters were varied over gamma distributions and probabilities and utilities were varied over beta distributions, with distributions for each parameter fitted to ranges shown in Supplementary Table 1. Additional analyses examined various assumptions regarding vaccine effectiveness against serotype 3 as well as potential increases in vaccine uptake occurring with the less complex PCV20 strategy.
Results
Public health results (cases, hospitalizations, and deaths) for Black and non-Black cohorts are shown in Table 1. Pneumococcal disease deaths prevented by vaccination were comparable, on a percentage basis, between vaccination strategies in both Black and non-Black cohorts. The Black cohort had an absolute 1.7% greater pneumococcal case-hospitalization risk compared to non-Black cohorts.
Base case cost-effectiveness results are summarized in Table 2. In the Black cohort, total per person PCV20 strategy costs, compared to no vaccination, were $124 greater while gaining 0.00073 QALYs, or $169,540/QALY gained while PCV15/PPSV23, compared to PCV20, cost $535,797/QALY gained. In the non-Black cohort, PCV20 cost $210,529/QALY gained compared to no vaccination and PCV15/PPSV23 cost $728,423/QALY compared to PCV20. Plausible variation of vaccine effectiveness based on CDC analysis-derived values had relatively minor effects on PCV20 cost-effectiveness, increasing cost/QALY gained by $8000–10,000 in either cohort. More substantial effects occurred in the cost-effectiveness of PCV15/PPSV23, with increases to more than $1 million per QALY gained compared to PCV20 due to assumptions of lower PPSV23 effectiveness in CDC-sponsored analyses.
Individual variation of parameter values in one-way sensitivity analyses had minimal effects on vaccination strategy favorability in both Black and non-Black analyses. In the Black cohort, a probabilistic sensitivity analysis varying all parameters simultaneously over distributions found that no vaccination was most likely to be favored at cost-effectiveness thresholds less than $180,000/QALY gained, while PCV20 was favored at thresholds from $180,000 to $400,000/QALY gained (Fig. 1). Probabilistic sensitivity analysis in the non-Black cohort had similar results, except that PCV20 was favored at thresholds greater than $190,000/QALY gained. In either cohort, the PCV15/PPSV23 strategy was unlikely to be favored (14% or 6% of model iterations in Black or non-Black cohorts, respectively, at a $200,000/QALY gained threshold).
Probabilistic sensitivity analysis of pneumococcal vaccine strategies in the 65-year-old Black cohort. Curves depict the likelihood of strategies being favored (y-axis) over a range of willingness-to-pay (or acceptability) thresholds (x-axis) when all parameters are varied simultaneously. PCV20 is favored at thresholds of $180,000 per QALY gained or greater, while no vaccination is favored at lower thresholds. PCV15 = 15-valent pneumococcal conjugate vaccine, PCV20 = 20-valent pneumococcal conjugate vaccine, PPSV23 = 23-valent pneumococcal polysaccharide vaccine, QALY = quality-adjusted life year
In scenarios where the simpler one-vaccine PCV20 strategy increased the likelihood of absolute vaccine uptake by 10% (from 59.8% to 69.8% in the Black cohort), PCV20 cost-effectiveness changed minimally while PCV15/PPSV23 cost more than $3 million/QALY gained in the Black and non-Black cohort analyses (Table 3, middle column). These results were due to absolute changes in incremental effectiveness being smaller, on a relative scale, for PCV20 and greater for PCV15/PPSV23 (Supplementary Table 3).
We also examined additional scenarios to address concerns that PCV20 may be less effective than PCV15 owing to either serotype-specific immunity decreasing as a result of more added serotypes or inherently less effectiveness against serotype 3. In a scenario where added serotypes decreased PCV20 effectiveness against all its component serotypes, its relative effectiveness would need to decrease at least 15% for PCV20 to become unfavorable in either population group (Table 3, right column), and further illustrated in Fig. 2 (top panel). In another scenario where PCV20 effectiveness against serotype 3 is less than that of PCV15, PCV20 continued to be favored compared to PCV15/PPSV23 throughout the range of potential decreased effectiveness values (Fig. 2, bottom panel). Finally, modeling scenarios for increased effectiveness for all conjugate vaccines against serotype 3, using ranges consistent with CDC analyses, did not materially change analysis results.
Sensitivity analysis: scenarios with decreased PCV20 effectiveness relative to PCV15 in the Black 65-year-old cohort. Panels depict changes in vaccine strategy cost-effectiveness when PCV20 effectiveness is decreased against all its component serotypes (top panel) or against pneumococcal serotype 3 (bottom panel). In the top panel, when PCV20 has a higher ICER than PCV15/PPSV23, PCV20 is also less effective than PCV15/PPSV23; thus, PCV20 is thus extended dominated in this circumstance. ICER = incremental cost-effectiveness ratio, PCV15 = 15-valent pneumococcal conjugate vaccine, PCV20 = 20-valent pneumococcal conjugate vaccine, PPSV23 = 23-valent pneumococcal polysaccharide vaccine, QALY = quality-adjusted life year
Discussion
In this decision analysis examining recently revised recommendations for pneumococcal vaccination in adults aged 65 years and older, we found that newly recommended strategies using either PCV20 or PCV15/PPSV23 were equally effective in preventing pneumococcal disease deaths in both Black and non-Black populations and that pneumococcal disease hospitalization risk was greater in Black older adult populations, confirming our prior work. However, the single-dose PCV20 strategy was economically favorable to the two-dose PCV15/PPSV23 strategy in both Black and non-Black populations in the base case analysis. The PCV20 strategy was more favorable in Black populations, with this finding remaining robust to variation of several vaccine effectiveness assumptions. In addition, if the less complex PCV20 strategy increases vaccine uptake compared to the PCV15/PPSV23 strategy, which could occur in populations with barriers to healthcare access, then PCV15/PPSV23 became substantially more economically unfavorable compared to PCV20. Increased uptake could result from the PCV20 strategy being more attractive or more accessible to clinicians and patients, increasing vaccination frequency, or due to incompletion of the PCV15/PPSV23 sequence as a result of non-receipt of PPSV23 after 1 year.
Medically underserved populations are at greater risk for pneumococcal disease, largely because of greater likelihood of chronic medical conditions that increase risk and lower vaccination rates resulting from decreased access to vaccination. Similar trends are seen in Black populations, which we used in this analysis as a proxy for the medically underserved. Policy equity and the potential for policies to decrease disparities are now considerations in CDC vaccination recommendation deliberations [1]. On the basis of our results, PCV20 use in the general population of older adults appears to be a more justifiable strategy to address and mitigate racial disparities in pneumococcal disease, owing to the potential for greater impact on Black populations compared to PCV15/PPSV23.
Prior analyses show that childhood pneumococcal vaccination substantially decreases the cost-effectiveness of adult pneumococcal vaccination owing to indirect (herd protection) effects of childhood conjugate vaccine usage [14]. Our analysis does not account for these effects from future PCV15 and PCV20 use in children, a limitation of our study. Evaluation of those vaccines for childhood use is ongoing, with approval for PCV15 expected first (in 2022), followed later by PCV20. In the shorter term, these approvals may more substantially decrease PCV15/PPSV23 favorability in older adults if PCV15 is approved first for children. In the longer term, reconsideration of recommendations for adult conjugate vaccine use may be necessary if childhood vaccination with PCV15 or PCV20 has similar indirect effects to those seen previously with PCV13, where adult disease due to PCV13 serotypes significantly decreased soon after childhood PCV13 use began.
This analysis is also limited by some simplifying assumptions and uncertainty in key parameter values. Prior adult vaccination strategies have not resulted in substantial decreases in disease due to pneumococcal serotype 3 [12]. It is not clear if the newer vaccines will have increased effectiveness against serotype 3, or if one of the newer vaccines will be more effective against serotype 3 than the other. To address this uncertainty, we examined variable effectiveness of both vaccines and differential effectiveness between vaccines (i.e., increased effectiveness of PCV15) against serotype 3 [15]; we found that plausible variation did not substantially change our findings. Another area of uncertainly is PPSV23 effectiveness against NBP. Our previous analyses, for the most part, assumed little or no effectiveness. However, more recent data suggest some PPSV23 effectiveness against NBP, but still less than that seen with conjugate vaccines [16]. Greater PPSV23 effectiveness would improve the favorability of PCV15/PPSV23. However, our assumption that all PCV15 vaccinated individuals receive PPSV23 a year later likely biases results in favor of the PCV15/PPSV23 strategy; relaxing this assumption would make this strategy less favorable. In addition, our NBP rate estimates could be considered conservatively low. If NBP rates are higher, then incremental cost-effectiveness ratios for both newly recommended strategies will be lower.
We only considered newly recommended vaccination strategies in this analysis. The prior recommendation was not included in the analysis because PCV13 use in adults is no longer recommended in the USA and recent analyses found the prior recommendation more expensive and less effective than other options [4]. Future vaccines that prevent more illness or modified vaccination strategies that result in greater vaccine uptake were not modeled. Interventions that increase vaccine uptake could be economically reasonable and have favorable public health impact [7]. Routine pneumococcal vaccination for all adults at age 50 has been considered [1]; if adopted, impact on vaccination in older adults would be unclear. Finally, in a scenario analysis, we speculate that a single-dose PCV20 strategy has greater implementation feasibility than the two-dose PCV15/PPSV23 strategy. Future work will include data on documented changes in vaccine uptake observed after these newly revised pneumococcal vaccination recommendations have been in place.
Conclusions
With these limitations in mind, we conclude that, of the newly recommended adult pneumococcal vaccination strategies, PCV20 use is substantially more economically reasonable in Black and non-Black older adult populations compared to PCV15/PPSV23. If using a single vaccine increases uptake compared to a more complex two-vaccine strategy, which is potentially more likely in the underserved, then a PCV20 strategy becomes even more favorable.
References
Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–117.
Kobayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among U.S. adults and proposed policy options. ACIP meeting Pneumococcal Vaccines 2021; https://stacks.cdc.gov/view/cdc/110908. Accessed 10 Feb 2022.
Stoecker C. Economic assessment of PCV15 & PCV20. ACIP meeting Pneumococcal Vaccines 2021; https://stacks.cdc.gov/view/cdc/109109. Accessed 10 Feb 2022.
Leidner AJ. Summary of three economic models assessing pneumococcal vaccines in US adults. ACIP meeting Pneumococcal Vaccines 2021; https://stacks.cdc.gov/view/cdc/110717. Accessed 10 Feb 2022.
Nowalk MP, Wateska AR, Lin CJ, et al. Racial disparities in adult pneumococcal vaccination indications and pneumococcal hospitalizations in the U.S. J Natl Med Assoc. 2019;111(5):540–5.
Wateska AR, Nowalk MP, Lin CJ, et al. Pneumococcal vaccination in adults aged ≥65 years: cost-effectiveness and health impact in U.S. populations. Am J Prev Med. 2020;58(4):487–495.
Wateska AR, Nowalk MP, Lin CJ, et al. Cost-effectiveness of pneumococcal vaccination policies and uptake programs in US older populations. J Am Geriatr Soc. 2020;68(6):1271–8.
Zimmerman RK, Brown AE, Pavlik VN, et al. Using the 4 pillars practice transformation program to increase pneumococcal immunizations for older adults: a cluster-randomized trial. J Am Geriatr Soc. 2017;65(1):114–22.
US Census Bureau. https://www2.census.gov/programs-surveys/popest/datasets/2010-2020/national/asrh/. Accessed 10 Feb 2022. File: NC-EST2020-ALLDATA-P-File24.csv.
Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547–8.
CDC. CDC vacccine price list. www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html. Accessed 10 Feb 2022.
Pilishvili T. 13-valent pneumococcal conjugate vaccine (PCV13) effects on disease caused by serotype 3. ACIP meeting Pneumococcal Vaccines 2019; https://stacks.cdc.gov/view/cdc/78091. Accessed 10 Feb 2022.
Said MA, Johnson HL, Nonyane BAS, Deloria-Knoll M, O’Brien KL. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4): e60273.
Smith KJ, Wateska AR, Nowalk MP, et al. Higher-valency pneumococcal conjugate vaccines: an exploratory cost-effectiveness analysis in U.S. seniors. Am J Prev Med. 2021;61(1):28–36.
Buchwald UK. V114: an investigational 15-valent pneumococcal polysaccharide conjugate vaccine (PCV) : key results of the adult clinical development program. ACIP meeting Pneumococcal Vaccines 2021. https://stacks.cdc.gov/view/cdc/107069. Accessed 10 Feb 2022.
Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 2020;17(10): e1003326.
Acknowlegements
Funding
The study and the journal’s Rapid Service Fee were supported by the National Institute of Allergy and Infectious Diseases (grant number R01 AI11657503).
Author Contributions
Angela R. Wateska and Kenneth J. Smith performed the analysis, based on a model they built with input from Richard K. Zimmerman, Mary Patricia Nowalk, Chyongchiou J. Lin, Lee H. Harrison, and William Schaffner. Angela R. Wateska and Kenneth J. Smith drafted the manuscript with critical revision by Richard K. Zimmerman, Mary Patricia Nowalk, Chyongchiou J. Lin, Lee H. Harrison, and William Schaffner. All authors read and approved the final manuscript.
Disclosures
Kenneth J. Smith and Richard K. Zimmerman have an active research grant from Sanofi Pasteur on an unrelated topic. Chyongchiou J. Lin and Mary Patricia Nowalk have grant funding from Merck & Co., Inc. on an unrelated topic and had research grants within 3 years from Pfizer, Inc. and Sanofi Pasteur on unrelated topics that are no longer active. William Schaffner is a member of a data safety monitoring board (DSMB) for Pfizer, former member of a DSMB for Merck, and has served as a consultant to Roche Diagnostics. Lee H. Harrison has served as a consultant to GSK, Merck, Pfizer, and Sanofi Pasteur. Angela R. Wateska has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on data from previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Smith, K.J., Wateska, A.R., Nowalk, M.P. et al. Cost-Effectiveness of Newly Recommended Pneumococcal Vaccination Strategies in Older Underserved Minority Adults in the USA. Infect Dis Ther 11, 1683–1693 (2022). https://doi.org/10.1007/s40121-022-00669-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00669-x