Abstract
Introduction
Ledipasvir/sofosbuvir (LDV/SOF) for hepatitis C virus (HCV) treatment provides an oral interferon-free treatment regimen with high rates of sustained virologic response (SVR). This study assessed treatment discontinuation, factors associated with treatment completion, and real-world effectiveness.
Methods
Patients with HCV treated with LDV/SOF between October 2014 and June 2015 and enrolled in a large US health plan were identified. Expected treatment duration was calculated based on IDSA/AASLD treatment guidelines and US labels using data for genotype, initial treatment regimen, baseline cirrhosis, and prior treatments. Logistic regression was used to identify factors associated with treatment completion, controlling for patient characteristics.
Results
The study included 1483 LDV/SOF patients. Mean age was 59.7 years, most were male (63.9%), had commercial insurance (51.9%), and were treatment-naïve (85.6%). Cirrhosis or end stage liver disease was present in 46.1%. Among patients with an expected 8-week treatment regimen, 49.4% were treated for longer. Most patients (99.8%) with expected 12-week treatment durations were adherent to the expected treatment duration. Treatment-experienced patients [odds ratio (OR) 0.124, p < 0.001] and those on Medicare (OR 0.382, p = 0.039) had lower odds of completing the expected treatment regimen, while males were more likely to complete treatment than females (OR 3.235, p = 0.003). SVR12 in patients treated with LDV/SOF was 89.4% (n = 76/85).
Conclusion
Half of patients eligible for an 8-week treatment regimen with LDV/SOF were treated longer, while most patients with a 12-week regimen were adherent to the expected treatment duration. Prior HCV treatment, female gender, and Medicare Advantage insurance were associated with lower odds of treatment completion. Overall SVR12 was 89.4%.
Funding
Merck & Co. Inc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 3.6 million people in the US have hepatitis C virus (HCV) antibodies, with 2.7 million currently infected [1]. Accounting for high-risk populations, the estimate is likely much higher, with at least 4.6 million carrying HCV antibodies and 3.5 million currently infected [2]. Hepatitis C is the leading cause of liver transplantation and hepatocellular cancer and is associated with increased mortality [3]. Early HCV infections are often asymptomatic or present with non-specific symptoms; thus, most infections are diagnosed after serious liver damage has already occurred. The goal of treatment is sustained virologic response (SVR), which represents a virologic cure and is associated with a reduced risk of mortality, cirrhosis, and hepatocellular cancer [4, 5]. SVR has also been shown to reverse fibrosis and significantly reduce liver complications in compensated and decompensated cirrhosis [6, 7].
HCV is genetically heterogeneous with at least six genotypes. Genotype 1 is the most prevalent, representing approximately 76% of infections in the US and 59–89% in Europe [8]. Preliminary guidelines for treatment regimen are based on genotype, with some therapies initially approved with genotype-restricted activity [9]. SVR rates vary based on patient adherence, treatment experience, disease severity, and a variety of other known (comorbidities) and unknown (genomic) host factors.
Historically, treatment consisted of weekly injections of pegylated interferon plus ribavirin (RBV) for 48 weeks, yielding SVRs of 45–50%. Direct-acting antiviral agents (DAAs) were introduced in 2011. In combination with pegylated interferon and RBV, SVR rates rose to 67–73% in treatment naïve, genotype 1 patients. Additional DAAs were approved in 2013, including the RNA-dependent RNA polymerase nonstructural protein 5B inhibitor, sofosbuvir (SOF). In 2014, the FDA approved the first fixed-dose oral combination therapy, ledipasvir (NS5A inhibitor)/sofosbuvir (LDV/SOF), for the treatment of patients with HCV genotype 1 and, more recently, genotypes 4, 5, and 6. In clinical trials, treatment with LDV/SOF resulted in SVRs of ≥97% over 12 and 24 weeks [10] and ≥93% over 8 weeks [11]. In patients with previous interferon-based therapy failure, ≥94% achieved SVR with LDV/SOF, compared to a historical rate of 25% [12]. Shortly after the approval of LDV/SOF, the FDA approved ombitasvir/paritaprevir/ritonavir plus dasabuvir, which may require co-administration of RBV [13]. While this regimen offers similar efficacy, the advantage of LDV/SOF is once-daily dosing that does not require RBV co-administration.
In December 2014, LDV/SOF was added to the HCV treatment guidelines (http://www.hcvguidelines.org/full-report-view). Recommended treatment for genotype 1, non-cirrhotic, treatment-naïve patients is 12 weeks; however, patients with baseline HCV RNA <6MM IU/mL may be treated for 8 weeks. In cirrhotic patients, recommendations are based on prior treatment. Twelve weeks of LDV/SOF are recommended for treatment-naive patients and 24 weeks are recommended for patients with prior treatment failure [14].
Data on adherence to and discontinuation of LDV/SOF in clinical practice are limited. Patients treated in real-world clinical settings are more likely to have complex disease manifestation than those who participate in clinical trials. It is important to understand the real-world utilization of LDV/SOF and to assess adherence in order to develop strategies to increase efficacy of these regimens in clinical practice and to identify patients at risk for adherence-related treatment failure.
The objectives of this study were to assess discontinuation, adherence to the expected treatment duration, and factors associated with discontinuation in patients receiving LDV/SOF. Additionally, characteristics of patients who received treatment were evaluated and SVR was assessed.
Methods
Study Design and Data Source
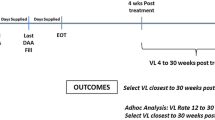
This was a retrospective study using medical and pharmacy claims data, enrollment information, and linked laboratory results from two administrative health plan databases, the Optum Research Database and Impact National Benchmark Database (Impact). Data extracted for each patient spanned November 1, 2013 through June 30, 2015 (Fig. 1). Medical claims data included International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Healthcare Common Procedure Coding System codes, and revenue codes. Pharmacy claims data included National Drug Codes for filled prescriptions, days supplied, and quantity of drug supplied. Linked outpatient laboratory results were available for a subset of the research database. This article does not contain any new studies with human or animal subjects performed by any of the authors and no identifiable protected health information was extracted or accessed during the course of the study. Pursuant to the Health Insurance Portability and Accountability Act, the use of de-identified data did not require Institutional Review Board approval or waiver of authorization.
Study Population
To be included in the study, patients were required to have ≥1 pharmacy claim for LDV/SOF from October 10, 2014 (date of FDA approval) through June 30, 2015. The index date was defined as the first pharmacy claim for LDV/SOF during the patient identification period. For patients with more than one treatment regimen, the last regimen was selected for analysis. Patients were required to have continuous enrollment with medical and pharmacy coverage at least 12 months prior to the index date and until treatment discontinuation (defined as a gap in medication possession of 30 days for the first gap and 14 days for any subsequent gaps) and be at least 18 years old. The initial gap of 30 days was selected as a conservative estimate of a potential delay between the date of the first fill and first administration. The subsequent gap of 14 days was selected as a maximum permissible period without therapy beyond which a patient could anticipate reduced or suboptimal outcomes [15]. The post-treatment period was initiated based on the run-out of the last prescription fill. Patients with interferon use during the treatment period were excluded from the study.
Study Measures
Patient Characteristics
Age, gender, geographic location, health plan type (commercial, Medicare Advantage), length of pre-index period, and prescribing physician specialty were reported as of the index date. The Quan–Charlson comorbidity score and liver severity algorithm [16] were calculated during the pre-index period. The presence of clinically relevant conditions/events (including advanced liver disease and cirrhosis) during the pre-index period was identified based on ICD-9-CM diagnosis and procedure codes from pre-index medical claims. Treatment status was determined by pharmacy and medical claims and categorized as the presence/absence of any medication.
Clinical Characteristics
The following laboratory results were measured during the baseline period to detect liver damage and fibrosis: alanine aminotransferase, aspartate aminotransferase, and platelet count. The aspartate aminotransferase-to-platelet ratio index (APRI) was classified as no/minimal fibrosis, <0.5; moderate fibrosis, 0.5–1.5; and significant fibrosis, >1.5. Using the aspartate aminotransferase, alanine aminotransferase, and platelet count results, fibrosis-4 (FIB-4) was calculated and defined as minimal fibrosis, <1.45, moderate fibrosis, 1.45–3.25, and significant fibrosis, >3.25 [17, 18]. The laboratory result closest to the index date was used for analysis. All HCV RNA test results during the study period were captured. HCV genotype and subtype were captured for those with available laboratory results. If multiple genotypes were recorded, the last genotype result measured during the study period was used.
Treatment Completion and Adherence to Expected Treatment Duration
Treatment completion was defined as having completed a full course of treatment as per IDSA/AASLD treatment guidelines and the Food and Drug Administration-approved labeling for the regimen [19]. Adherence to the expected treatment duration was evaluated by comparing the actual treatment length to the expected treatment duration. The expected treatment duration was calculated based on IDSA/AASLD treatment guidelines accounting for HCV genotype, HCV RNA level, treatment status (naïve or experienced for ≥1 year), and baseline cirrhosis diagnosis [19]. If genotype data were unavailable, the expected duration was calculated from the observed treatment duration, treatment regimen, cirrhosis diagnosis, and treatment status (naïve or experienced). The observed treatment duration was calculated from the total number of days with medication supply for LDV/SOF until discontinuation.
Sustained Virologic Response
SVR is defined as the proportion of patients with HCV RNA below the limit of quantification performed ≥12 weeks after treatment completion (SVR12). When SVR12 was not available, HCV RNA was captured from 8 weeks post-treatment completion to maximize the number of available results. Studies have shown high concordance with a viral load measured between 4 and 12 weeks [20,21,22].
Statistical Analyses
Descriptive statistics were used to describe the distribution of demographics and clinical and laboratory characteristics. Logistic regression was used to model expected treatment completion, adjusting for demographics (age, gender, insurance type), comorbidities (Quan–Charlson comorbidity score, cirrhosis, chronic kidney disease, HIV, diabetes, cardiovascular disease, hypertension, hepatitis B, drug abuse, and liver transplant), and prior HCV treatment. The covariance matrix was examined to diagnose collinearity in the model.
Results
After inclusion and exclusion criteria were applied, 3438 patients receiving oral DAAs remained; of these, 1483 received LDV/SOF (Fig. 2). More than half (54.2%) of patients had at least 3 years of cumulative pre-index observation time to assess baseline characteristics. Patient age averaged 59.7 years and most were male (63.9%), had commercial insurance (51.9%), and were treatment-naïve (85.6%). Of the 39.8% of patients with a known genotype, 98.8% had genotype 1 (Table 1). Cirrhosis or end stage liver disease was present in 46.1% of patients, HIV/AIDS in 7.6%, and 35.0% and 32.6% had evidence of depression or anxiety, respectively. Diagnosis codes suggesting history of drug abuse were found for 21.2% of patients. In patients with valid APRI (n = 674) and FIB-4 (n = 675) results, 60.7% and 71.6% of patients, respectively, had moderate to severe fibrosis (Table 2).
Among patients eligible for an 8-week treatment duration, 49.4% were treated longer. In contrast, the majority of patients with a 12- and 24-week expected treatment duration were adherent to the expected duration (99.8% and 74.6%, respectively) (Table 3). Of 1100 patients with known expected treatment duration, the overall early discontinuation rate was 3.0% (1.7% for patients with expected treatment duration of 8 weeks and 0.3% for patients with expected treatment duration of 12 weeks), but increased with longer expected treatment duration (25.5% for patients with expected treatment duration of 24 weeks). In patients who discontinued early, side effects (anemia, rash, gastrointestinal symptoms, headache, fatigue, or insomnia) were noted in 39.4% in the 4 weeks prior to treatment discontinuation (data not shown). Additionally, 49.4% of those eligible for an 8-week treatment duration extended beyond the 8 weeks, while no one with an expected duration of 12 or 24 weeks extended treatment.
A logistic regression model of early treatment discontinuation showed that the odds of completing treatment were lower in those with prior HCV treatment [odds ratio (OR) 0.12, p < 0.001] and those with Medicare Advantage coverage (OR 0.38, p = 0.039), while males had over 3 times higher odds of completing treatment without early discontinuation than females (OR 3.24, p = 0.003) (Table 4). A diagnosis of cirrhosis was not statistically significant (0.46, p = 0.060), nor were other demographics and comorbidities.
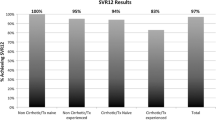
In the 85 patients with complete and valid laboratory results who completed HCV treatment, overall SVR was 89.4% (Table 5). SVR was highest among patients with an observed treatment duration of 12 weeks (92.9%, 65/70) and lower for those with an 8-week (75.0%, 9/12) regimen. Additionally, slightly fewer patients with cirrhosis reached SVR (85.0%, n = 17/20) than patients without cirrhosis (90.8%, n = 59/65), while those with prior treatment (90.0%, n = 9/10) had a similar SVR to those who were treatment naïve (89.3%, n = 67/75). Although laboratory results were only available for a small subset of the patient population, baseline demographic and clinical characteristics were similar between those with and without SVR results (Supplementary Table 1).
Discussion
This retrospective study evaluated patient characteristics and medication discontinuation and adherence to LDV/SOF in HCV genotype 1 patients in the US. Our results provide important insights into adherence to and discontinuation of LDV/SOF in patients with HCV in a real-world setting. Adherence to the expected treatment duration was over 99% in those with a 12-week regimen. Those with a 24-week treatment duration were more likely to experience early treatment discontinuation, and almost half of the patients who were eligible for 8 weeks of treatment were treated longer.
The latter findings were similar to other recent real-world studies. In a study population from the TRIO network, 50% of those eligible for 8 weeks of treatment were treated longer [23]. Similarly, two additional real-world studies reported that 42% [24] and 60% [25] of patients who were eligible for 8 weeks of treatment were treated for 12 weeks. These results suggest that patients were prescribed treatment for a longer duration than recommended in the product labeling. It is important to note the limitation that, while HIV/AIDS status impacts the expected treatment duration, it was not accounted for in our analysis.
The overall early discontinuation rate was 3.0%. Early discontinuation rates were 1.7% for those with 8-week and 0.3% for those with 12-week treatment durations; however, 25.5% of patients who were eligible for 24 weeks of treatment were treated less than 24 weeks. Patients who were treatment-experienced versus treatment-naive or with Medicare Advantage coverage versus commercial insurance had lower odds of completing treatment, while males were more likely (OR = 3.2) to complete treatment than females. In the HCV-TARGET study, a consortium of academic and community medical centers, the overall discontinuation rate in those treated with LDV/SOF ± RBV for 8, 12, or 24 weeks was 1.9% [25]. Additionally, only 1.5% of patients with cirrhosis in the TRIO cohort discontinued early during 24 weeks of treatment [23]. The higher discontinuation rate reported in our study for the 24-week patients could represent the eligibility for shorter treatment durations. Additionally, these patients have more severe and advanced disease and may experience more treatment-related side effects. In those who discontinued early, almost 40% had evidence of side effects (anemia, rash, gastrointestinal symptoms, headache, fatigue, or insomnia) in the 4 weeks prior to treatment discontinuation.
In clinical trials of LDV/SOF, SVR rates among genotype 1 HCV-infected patients ranged from 94% in those with previous treatment failure [12] to 97% in treatment-naïve patients [10]. Patients in real-world clinical settings are often sicker, older, have more comorbidities, and are not followed as closely as those in clinical trials. In our study, SVR in patients treated for 12 weeks was 93% (n = 65/70). Slightly fewer patients with cirrhosis (85%) reached SVR compared to patients without cirrhosis (91%), and there was no significant difference between treatment-experienced and treatment-naïve patients (89% vs. 90%). Overall, SVR was 89.4%. Our findings are consistent with findings from the Veterans Affairs population evaluating genotype1 patients with overall SVR rates of 91–92% in those treated with LDV/SOF [22, 26]. Similar to our study, treatment-experienced cirrhotic patients in the TRIO population had a SVR rate of 84% at 12 weeks [27]. Treatment-naïve patients in the TRIO network treated with LDV/SOF for 12 weeks also experienced similar rates of SVR12 as our study, with a rate of 94% [28].
The overall effectiveness rate of LDV/SOF in this study is consistent with large database studies especially in the Veterans Affairs population [22, 26]. In our study, patients had higher baseline cirrhosis or ESLD (46.8%) compared to that reported in other studies (ranged from 30% to 38%) [24,25,26, 28], which may have affected rates of SVR.
Limitations
Claims data provide a powerful method to examine utilization and effectiveness in routine clinical settings. These data also offer the advantage of large sample sizes of patients with diverse medical histories; however, certain limitations inherent to claims-based analyses should be considered when interpreting the results of this study. First, the presence of a medical or pharmacy claim is not proof positive for the presence of disease or that the medication was consumed or taken as prescribed. Second, laboratory test results were only available for a subset of patients. The extent of missing laboratory test results may not be distinguishable from the lack of an administered test. Additionally, misclassification of treatment naïve status could exist because the study required 1 year or more of baseline medical history; however, more than half of patients in this study had at least 3 years of baseline data. The lack of HIV/AIDS status may also have resulted in misclassification of the 8-week treatment regimen. The study data come from a commercial and Medicare Advantage population and may not be generalizable to the entire US population. Lastly, our inability to detect deaths, particularly in this advanced population, may have resulted in misclassification of some patients as early discontinuers.
Conclusion
Within the observed sample of a US insured population, the overall early discontinuation rate for patients treated with LDV/SOF was 3%. Half of patients with HCV who were eligible for an 8-week LDV/SOF regimen were treated longer, while most patients with a 12-week regimen were adherent to the expected treatment duration. Controlling for patient characteristics, those with prior HCV treatment, females, and those on Medicare Advantage were less likely to complete the expected amount of treatment. Overall SVR in patients treated with LDV/SOF was 89.4%. It is important to understand adherence and patient characteristics associated with treatment completion in real-world clinical practice to develop better strategies to increase efficacy in those settings.
References
Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, national health and nutrition examination survey 2003 to 2010. Ann Intern Med. 2014;160:293–300.
Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–63.
McHutchison JG, Bacon BR. Chronic hepatitis C: an age wave of disease burden. Am J Manag Care. 2005;11:S286–95 (quiz S307–211).
Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, Ascione A, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87.
Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, Chen CH, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13:409–14.
Crissien AM MW, Pan JJ, Waalen J, Frenette CT, Pockros PJ. Regression of advanced fibrosis or cirrhosis measured by elastography in patients with chronic hepatitis C who achieve sustained virologic response after treatment for HCV. In: Program and abstracts of the 66th annual meeting of the American Association for the Study of Liver Disease November 13–17, 2015; San Francisco, CA.
Saxena V NL, Dasgupta A, Straley S, Catalli L, Nyberg AH, Terrault N. Hepatitis C virus (HCV) eradication in patients with varying severity of cirrhosis: impact on portal hypertensive complications, liver transplant (LT) and death. In: Program and abstracts of the 66th annual meeting of the American Association for the study of Liver Disease November 13–17, 2015; San Francisco, CA.
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87.
Trivella JP, Gutierrez J, Martin P. Dasabuvir: a new direct antiviral agent for the treatment of hepatitis C. Expert Opin Pharmacother. 2015;16:617–24.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98.
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88.
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93.
AbbVie. Viekira Pak prescribing information. 2015. http://www.rxabbvie.com/pdf/viekirapak_pi.pdf. Accessed Dec 2015.
Bourliere M, Bronowicki JP, de Ledinghen V, Hezode C, Zoulim F, Mathurin P, Tran A, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015;15:397–404.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7.
Gordon SPP, Terrault N, Hoop R, Buikema A, Nerenz D, Hamzeh F. Impact of disease severity on health care costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology. 2012;56:1651–60.
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25.
Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6.
America IDSo. HCV guidance: recommendations for testing, managing, and treating hepatitis C. 2015. http://www.hcvguidelines.org/full-report-view. Accessed Nov 2015.
Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, Wang J, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41–5.
Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, Kugelmas M, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–97.
Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65:426–38.
Curry M BB, Dieterich D, Flamm S, Guest L, Kowdley K, Lee Y, Tsai N, Younossi Z. Effectiveness of 8 or 12 week LDV-SOF in treatment naive patients with non-cirrhotic, genotype 1 Hepatitis C: Real-world experience from the TRIO network. In: Program and abstracts of the 66th annual meeting of the American Association for the Study of Liver Disease November 13–17, 2015; San Francisco, CA.
Backus L BP, Shahoumian T, Loomis T. Effectiveness of ledipasvir/sofosbuvir in treatment naive genotype 1 patients treated in routine medical practice. In: Program and abstracts of the 66th annual meeting of the American Association for the Study of Liver Disease November 13–17, 2015; San Francisco, CA.
Terrault N ZS, Bisceglie A, Lim J, Pockros P, Frazier L, Kuo A, Lok A, Shiffman M, Ari Z, Stewart T, Sulkowski M, Fried M, Nelson D. Treatment outcomes with 8, 12, and 24 week regimens of ledipasvir/sofosbuvir for the treatment of hepatitis C infection: analysis of a multicenter prospective observational study. In: Program and abstracts of the 66th annual meeting of the American Association for the Study of Liver Disease November 13–17, 2015; San Francisco, CA.
Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405–14.
Curry M BB, Dieterich D, Flamm S, Guest L, Kowdley K, Lee Y, Tsai N, Younossi Z. Effectiveness of 12 or 24 week LDV-SOF and 12 week LDV-SOF + RBV in Treatment-experienced patients with cirrhotic, genotype 1 hepatitis C: real-world experience from the TRIO network. In: Program and abstracts of the 66th annual meeting of the American Association for the study of Liver Disease November 13–17, 2015; San Francisco, CA.
Afdhal N BB, Dieterich D, Flamm S, Guest L, Kowdley K, Lee Y, Tsai N, Younossi Z. Failure with all-oral DAA regimens: academic and community treatment of a real-world population from the TRIO network. In: Program and abstracts from the 66th annual meeting of the American Association for the Study of Liver Disease November 13–17, 2015; San Francisco, CA.
Acknowledgements
Sponsorship for this study and article processing charges was funded by Merck & Co. Michael Hull and Jeffrey McPheeters had access to all of the data in this study and all authors take responsibility for the integrity of the data and accuracy of the data analysis. Inc. Deja Scott-Shemon (Optum, Eden Prairie, MN) provided medical writing support for this manuscript, which was funded by Merck & Co. Inc. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Michael Hull is an employee of Optum, and was funded by Merck & Co., Inc. to conduct the study. Jeff McPheeters is an employee of Optum, and was funded by Merck & Co., Inc. to conduct the study. Dr. Kay Schwebke is an employee of Optum, and was funded by Merck & Co., Inc. to conduct the study. Dr. Puenpatom is an employee of Merck & Co., Inc.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors and no identifiable protected health information was extracted or accessed during the course of the study. Pursuant to the Health Insurance Portability and Accountability Act, the use of de-identified data did not require Institutional Review Board approval or waiver of authorization.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to the proprietary elements owned by Optum. The disclosure of this data to third party clients assumes certain data security and privacy protocols are in place and that the third party client has executed our standard license agreement which includes restrictive covenants governing the use of the data.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/5BD8F0601440B5CD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Puenpatom, A., Hull, M., McPheeters, J. et al. Treatment Discontinuation, Adherence, and Real-World Effectiveness Among Patients Treated with Ledipasvir/Sofosbuvir in the United States. Infect Dis Ther 6, 423–433 (2017). https://doi.org/10.1007/s40121-017-0163-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-017-0163-0