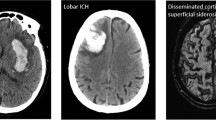
Abstract
Introduction
This study aimed to investigate the association between atrial fibrillation (AF), particularly newly diagnosed AF, and remote intracerebral hemorrhage (rICH) in patients with ischemic stroke who were treated with intravenous thrombolysis (IVT).
Methods
This observational study was conducted on patients with ischemic stroke who received IVT with recombinant tissue-type plasminogen activator. The data were taken from a multicenter prospective registry of a Chinese population. rICH was defined as any extraischemic hemorrhage detected on computerized tomography (CT) 24 h after intravenous thrombolysis. We collected and compared the demographic data and clinical characteristics of all the patients with rICH to those of patients without any type of hemorrhagic transformation. The association between AF and rICH was analyzed using univariate analysis and binary logistic regression.
Results
A total of 20,697 patients were included in the study, with 1566 (7.6%) experiencing intracerebral hemorrhage (ICH), 586 (2.8%) experiencing rICH, and 19,131 (92.4%) not experiencing any form of hemorrhagic transformation. Univariate analysis revealed significant differences in age, pre-thrombolysis systolic blood pressure, baseline National Institute of Health Stroke Scale score, previously known AF, newly diagnosed AF, coronary heart disease, congestive heart failure, hyperhomocysteinemia, and history of thrombolysis between the rICH and control groups (P < 0.05). Further multivariate logistic regression analysis indicated that total AF (OR 1.821, 95% CI 1.082–3.065, P < 0.05), previously known AF (OR 1.470, 95% CI 1.170–1.847), and newly diagnosed AF (OR 1.920, 95% CI 1.304–2.825) were independently associated with rICH.
Conclusions
This study suggests that AF, regardless of whether it is newly diagnosed or previously known, may be associated with the occurrence of rICH following intravenous thrombolysis. Interestingly, our findings suggest that newly diagnosed AF may have a stronger impact on rICH than previously known AF, although confirmation from more studies is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Remote intracerebral hemorrhage (rICH) is not uncommon and atrial fibrillation (AF) is often thought to be associated with the bleeding transformation in patients with acute ischemic stroke (AIS) after intravenous thrombolysis (IVT), so whether AF, especially untreated newly diagnosed AF, is associated with rICH remains unknown. |
What was learned from the study? |
We analyzed a cohort of 20,697 patients and found that AF was an independent risk factor for rICH. Of note, newly diagnosed AF had a more significant impact on rICH than previously known AF. |
It provides clinicians with an insight that, when administering IVT to patients with AF concomitant with AIS, as a result of an elevated risk of rICH, heightened vigilance toward alterations in the patients’ condition and preparation for pharmacological intervention or surgical procedures are essential. |
Introduction
Ischemic stroke is the most prevalent form of cerebrovascular disease and is characterized by significant morbidity, disability, and mortality. The use of intravenous recombinant tissue plasminogen activator (rt-PA) is widely recognized as one of the most effective treatments for ischemic stroke within the established time window, offering the potential for significant clinical benefit. However, intracerebral hemorrhage (ICH) remains a major concern, as it is the most serious complication of intravenous thrombolysis (IVT) in patients with acute ischemic stroke (AIS). The majority of ICH cases arise within or around areas of infarcted brain tissue, classified as hemorrhage transformation (HT), which may be due to disruption of the blood–brain barrier [1]. In some instances, ICH may occur in regions where there is no visible ischemic damage, classified as remote intracerebral hemorrhage (rICH) [2]. Alongside HT, the presence of rICH is also associated with adverse functional outcomes [3,4,5] and an increased risk of mortality [6]. Importantly, the underlying mechanism of rICH development may differ from that of other forms of HT. Consequently, identifying the risk factors associated with rICH in AIS treated with IVT may have significant clinical implications in terms of guiding the development of targeted prevention strategies.
Atrial fibrillation (AF) is a well-known risk factor for ischemic stroke, accounting for approximately 15–20% of ischemic stroke cases [7]. According to recent guidelines, patients with AF-associated strokes can benefit from IVT [8]. However, it has been established through previous studies that AF is associated with HT after IVT [9]. Of note, studies have reported that among patients with acute ischemic stroke and AF, 7.8–36.2% were diagnosed with AF for the first time after stroke [10,11,12]. The characteristics of newly diagnosed AF and previously known AF may differ, and their impact on rICH may also vary. To date, no study has examined the association between different types of AF (previously known AF and newly diagnosed AF) and rICH, and the association between AF and rICH is not completely understood [3, 13]. Therefore, the objective of this study was to investigate the relationship between different types of AF and rICH after IVT. We conducted a retrospective analysis of clinical data from a large multicenter sample of patients who underwent IVT in Zhejiang Province, China, to explore the potential association of AF with rICH in patients with ischemic stroke after IVT.
Methods
This was a multicenter, retrospective, observational study using data from a retrospective multicenter stroke registry, Computerbased Online Database of Acute Stroke Patients for Stroke Management Quality Evaluation (CASEII, NCT04487340) [14], which was conducted on patients with ischemic stroke who received intravenous (IV) rt-PA at 71 different hospitals in Zhejiang Province, China, between June 2017 and December 2021. The patients were consecutively included in the Zhejiang Stroke Medical Quality Control Center Platform, an academic institution based at the Second Affiliated Hospital of Zhejiang University School of Medicine, which monitors the quality of all reperfusion therapy cases performed at stroke centers in the region.
Dada Quality Control
As the Zhejiang Stroke Medical Quality Control Center, we have implemented a standardized medical record template to regulate the documentation process during stroke diagnosis and treatment across all stroke centers in Zhejiang province. Each center has dedicated personnel who receive specialized training from us. An automated chart data capture system subsequently scans the inpatient records of enrolled cases from each center, facilitating data extraction [14, 15]. Our trained investigators oversee the authenticity and reliability of this data. Moreover, we periodically assess a subset of the scanned medical records to ensure compliance with the record templates, maintain documentation consistency, and verify the precision of data extraction, making any required corrections.
Patients
The study included patients who were 18 years of age or older and received IV rt-PA within the first 4.5 h from the onset of symptoms. Patients who did not have documented brain computerized tomography (CT) results within the first 24 h after thrombolytic therapy or those who underwent endovascular therapy were excluded. Demographics such as age and sex, traditional vascular risk factors including smoking, hypertension, diabetes mellitus, hyperlipidemia, previous ischemic stroke, AF, coronary heart disease (CHD), congestive heart failure (CHF), and hyperhomocysteinemia (Hcy), previous medications such as antiplatelet agents, anticoagulants, and statins, blood pressure before thrombolysis, onset to treatment time (OTT), severity of neurological deficit as measured by the National Institutes of Health Stroke Scale (NIHSS) score, and etiology of stroke according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification were recorded. The identification of vascular risk factors was mainly through the collection of previous medical history. Intravenous thrombolytic therapy with rt-PA was administered with a dose of 0.9 mg/kg up to a maximum of 90 mg, with 10% of the total dose administered as a bolus and the rest infused over 1 h.
This study received approval from the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (2016 Annual Ethics Review No. 074). Written informed consent for IVT was obtained from all patients and their family members for relevant examination and treatment. Because patient information in the CASE-II was de-identified and anonymized before being released to the researchers, the informed consent requirement for this study was waived by the institutional review board.
Definition of AF
The study collected the patient’s past medical history, as well as an electrocardiogram and continuous electrocardiogram (ECG) monitoring for 24–48 h. AF was classified as either previously known AF or newly diagnosed AF. Previously known AF was defined as a previous history of AF according to the patient or medical recording systems. Newly diagnosed AF was defined as the absence of previous history of AF, but the detection of AF on ECG or ECG monitoring during the current hospital visit, regardless of whether the patient was in the emergency department or neurology department.
Definition of HT and rICH
In this study, HT was defined as the occurrence of new hemorrhage within infarcted brain tissue. HT was classified according to the Heidelberg bleeding classification [2], which includes two types: hemorrhagic infarct (HI) and parenchymal hemorrhage (PH). HI was further classified into two subtypes: HI1, which refers to small petechiae, and HI2, which refers to confluent petechiae. PH was divided into two subtypes: PH1, which involved less than 30% of the infarcted area with mild space-occupying effect, and PH2, which involved more than 30% of the infarcted area with an obvious mass effect. rICH was defined as ICH in a brain region without visible ischemic damage on 24-h CT scans, according to the Heidelberg bleeding classification [2].
Statistical Analysis
The patients in this study were divided into different groups based on bleeding complications, including rICH alone, rICH combined with HI/PH, and a control group without any HT. Demographic variables, traditional vascular risk factors, previous medications, baseline NIHSS score, blood pressure before thrombolysis, OTT, and stroke pathogenesis were compared between the rICH group and control group. Normally distributed measurement data were presented as the means ± SDs and compared using t tests, while nonnormally distributed data were expressed as medians (25th–75th percentile) and compared using the Mann–Whitney U test. Categorical data were expressed as numbers and percentages and compared using the χ2 test. Variables with a P value < 0.05 in univariate analysis were included in a multivariate logistic regression analysis model. SPSS version 22.0 was used for all statistical analyses, and a P value < 0.05 indicated statistical significance.
Results
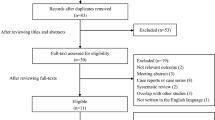
A total of 24,405 patients with ischemic stroke received reperfusion therapy (including IVT and endovascular therapy) during the study period. We excluded 1881 patients without a follow-up noncontrast CT within the first 24 h of IVT and 1827 patients who underwent endovascular therapy. Finally, 20,697 patients were included in the analysis (Fig. 1). Among the 20,697 patients, 1566 (7.6%) had ICH, and 19,131 (92.4%) did not experience any type of intracranial hemorrhage transformation (Table 1).
Univariate Analysis Results Comparing rICH and Control Groups
Table 2 presents the univariate analysis results comparing the baseline demographics, vascular risk factors, previous medications, and related indicators between the rICH and control groups prior to thrombolytic therapy. The rICH group exhibited a higher prevalence of total AF (29.9% vs. 15.9%, P < 0.001), previously known AF (24.6% vs. 13.5%, P < 0.001), and newly diagnosed AF (5.3% vs. 2.4%, P < 0.001) than the control group (Fig. 2). Additionally, patients in the rICH group were significantly older (mean age 74 years vs. 69 years; P < 0.001), had a higher systolic blood pressure before IVT (mean 156 mmHg vs. 153 mmHg; P = 0.002), and had a higher baseline NIHSS score (mean 7 vs. 4; P < 0.001). Furthermore, the rICH group had a higher prevalence of CHD (9.9% vs. 7.6%, P = 0.038), CHF (4.4% vs. 1.9%, P < 0.001), Hcy (9.9% vs. 6.6%, P = 0.002), and cardioembolism (31.9% vs. 16.4%, P < 0.001) compared to the control group. In addition, more patients in the rICH group had a history of thrombolysis (2.7% vs. 1.4%, P = 0.008). The rICH rates across the subtypes of the TOAST classification, encompassing cardioembolism (CE), stroke of undetermined cause (UND), large-artery atherosclerosis (LAA), small-artery occlusion (SAO), and stroke of other determined cause (ODC), are delineated in Fig. 3. The data revealed that the rICH rate in the UND group aligns more with CE than with LAA.
Multivariate Analysis Results for rICH After IVT
As presented in Fig. 4, multivariate logistic regression analysis was performed to adjust for covariance and determine the independent associations between various factors and the occurrence of rICH following IVT in patients with ischemic stroke. The analysis revealed that previously known AF (OR 1.470, 95% CI 1.170–1.847), newly diagnosed AF (OR 1.920, 95% CI 1.304–2.825), history of thrombolysis (OR 1.821, 95% CI 1.082–3.065), age (OR 1.021, 95% CI 1.013–1.029), history of CHF (OR 1.716, 95% CI 1.101–2.676), systolic blood pressure before thrombolysis (OR 1.005, 95% CI 1.001–1.009), and baseline NIHSS score (OR 1.027, 95% CI 1.016–1.038) were independently associated with the occurrence of rICH.
Multivariate analysis results for rICH after IVT. Final model of the multiple logistic regression analysis. OR odds ratio, CI confidence interval, Hcy hyperhomocysteinemia, AF atrial fibrillation, CHD coronary heart disease, CHF congestive heart failure, NIHSS National Institutes of Health Stroke Scale, OTT onset to treatment time
Univariate Analysis Comparing Newly Diagnosed AF with Previously Known AF Groups
Table 3 presents the results of a univariate analysis comparing the characteristics of patients with newly diagnosed AF versus those with previously known AF. The results revealed that the newly diagnosed AF group had a higher proportion of male patients (58.2% vs. 52.0%, P = 0.012), smokers (25.3% vs. 20.4%, P = 0.014), and individuals with elevated Hcy levels (11.4% vs. 8.2%, P = 0.018) than the previously known AF group. Additionally, the newly diagnosed AF group had significantly higher systolic (153 mmHg vs. 151 mmHg, P = 0.043) and diastolic blood pressures (86 mmHg vs. 85 mmHg, P = 0.013) before thrombolysis and lower rates of previous ischemic stroke (13.3% vs. 17.4%, P = 0.024), history of thrombolysis (1.2% vs. 3.1%, P = 0.021), CHD (9.4% vs. 23.6%, P < 0.001), CHF (2.2% vs. 9.5%, P < 0.001), previous antiplatelet therapy (13.5% vs. 26.7%, P < 0.001) and statin therapy (11.0% vs. 16.6%, P = 0.002) than the previously known AF group.
Discussion
Our findings revealed that approximately 2.8% of patients developed rICH, which is consistent with previous research. The National Institute of Neurological Disorders and Stroke in the USA reported an incidence of 1.3% for rICH [16]. The European Collaborative Acute Stroke Study (ECASS) and ECASS II found incidences of 3.7% and 2.0%, respectively [17]. These results align with previous findings, suggesting that rICH is not a rare condition.
Prior research has suggested that AF is linked to the occurrence of HT [9]. This may be due to several factors, including the ease with which AF can cause acute occlusion of large arteries, poor collateral circulation, and prolonged recanalization time [18, 19]. Previous studies have associated AF with PH but not rICH, recent work by Prats-Sánchez et al. [13] demonstrated an association between AF and rICH. In our study, we found that AF was an independent risk factor for rICH. The precise mechanism by which AF increases the risk of rICH remains unclear. However, based on a review of the available literature, we speculate that several factors may be at play. First, patients with AF frequently have a history of prior stroke or recent silent brain infarction, and these pre-existing vascular lesions have been linked to rICH [4]. A small prospective study by Drelon et al. [4] suggested that 41% (17/41) of rICH cases occurred in previously identified lesions confirmed by MR before thrombolysis. Second, AF-associated cardioembolism is associated with a greater burden of small vessel disease, such as white matter hyperintensities (WMHs) and cerebral microbleeds (CMBs) [20], which have been significantly linked to a higher risk of rICH in previous research [4, 21,22,23].
In this study, we found that approximately 15.3% (490/3209, Table 3) of patients with AF were newly diagnosed, and newly diagnosed AF (OR 1.920) a greater impact on rICH than previously known AF (OR 1.470). The newly diagnosed AF group had a higher proportion of males and smokers with higher systolic and diastolic blood pressures prior to thrombolysis than the previously known AF group. However, they were less likely to receive antiplatelet, anticoagulant, and statin therapy. The reason for this association remains unclear. We do know that antiplatelet, anticoagulant, and statin therapy can reduce the risk of asymptomatic cerebral infarction in patients with previously known AF [24]. In studies of newly diagnosed AF, administering antiplatelet or anticoagulant therapy resulted in a significant decrease in the risk of embolic events [25, 26]. Therefore, we hypothesize that patients with newly diagnosed AF are more likely to have a recent silent brain infarction before treatment than those with previously known AF, especially if they have not received antithrombotic therapy. As we mentioned earlier, recent silent brain infarction is associated with rICH [4]. Our findings suggest that patients with acute ischemic stroke combined with newly diagnosed AF receiving intravenous thrombolytic therapy have a higher risk of rICH. This association alerts clinicians to adopt a more proactive approach in screening for AF during patient management or health check-ups, and to implement appropriate primary prevention strategies. Not only can this mitigate the incidence of stroke, but it also can attenuate the risk of rICH occurring during thrombolysis at the time of AIS onset. Moreover, it provides clinicians with an insight that, when administering thrombolysis to patients with AF concomitant with AIS, especially those with newly diagnosed AF, as a result of an elevated risk of rICH, heightened vigilance toward alterations in the patients’ condition and preparation for pharmacological intervention (such as transfusion of cryoprecipitate) or surgical procedures are essential.
In addition, the reasons for the patient’s newly diagnosed atrial fibrillation, we postulate the following explanations: Firstly, the patient might not have previously undergone an ECG. Aside from residents or employees who receive annual health checkups, those from economically disadvantaged backgrounds or individuals who are not proactive about their health, especially those with asymptomatic AF, might not have sought an ECG evaluation. Secondly, the patient may have had paroxysmal atrial fibrillation that went undetected in prior ECG examinations. Lastly, the reason could be that the patient developed AF immediately prior to the stroke, or perhaps just a few days before the incident, making it virtually undetectable in regular health checks prior to the event.
In this study, we found that in 284 patients, a history of receiving at least two thrombolyses (OR 1.821, 95% CI 1.082–3.065) may be an independent risk factor for rICH. Most of the previous literature on repeated thrombolysis consists of case reports or small sample case series, showing that repeated thrombolysis has no significant effect on the occurrence of HT after IVT [27, 28]. Our study suggests that repeated thrombolysis may increase the risk of rICH. Previous studies have observed that intravenous thrombolytic therapy itself could increase intracranial microbleeds [29, 30], which were associated with the development of rICH after intravenous thrombolytic therapy [31]. Additionally, pre-existing blood–brain barrier disruption may be exacerbated after intravenous thrombolysis with alteplase [32], further contributing to the risk of rICH. The exact mechanism of this association remains unclear and requires further investigation. Additionally, our study found that age, a history of congestive heart failure, NIHSS score before thrombolysis, and systolic blood pressure before thrombolysis were independent factors associated with rICH. However, we did not find an association between onset-to-treatment time and rICH.
Notably, our study has some limitations. First, it was a retrospective case–control study, although the data collection and registration were conducted prospectively. Second, we did not include imaging features before and after thrombolysis, such as high-density shadow of the middle cerebral artery on CT, previously damage lesions, cerebral microbleeds, and white matter lesions. These factors could have helped us to better understand the underlying mechanisms contributing the association of AF and previous thrombolysis with rICH. Therefore, further research on these factors is needed.
Conclusions
In this study, we established that both newly diagnosed AF and previously known AF were independently associated with rICH, with newly diagnosed AF having a greater impact on rICH than previously known AF. These results could be instrumental in selecting suitable patients for future studies aimed at improving the safety of IVT.
Data Availability
Data are available upon reasonable request. The raw data supporting the conclusions of this article will be made available by the authors on reasonable request, without undue reservation.
References
Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30(11):2280–4.
von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–6.
Mazya MV, Ahmed N, Ford GA, et al. Remote or extraischemic intracerebral hemorrhage–an uncommon complication of stroke thrombolysis: results from the safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke. 2014;45(6):1657–63.
Drelon A, Kuchcinski G, Caparros F, et al. Remote brain hemorrhage after IV thrombolysis: role of preexisting lesions. Neurology. 2020;94(9):e961–7.
Martínez-Hernández E, Martínez-Ramírez S, Delgado-Mederos R, et al. Remote cerebral hematomas in patients treated with intravenous rt-PA. J Neurol. 2010;257(7):1062–6.
Qiu L, Fu F, Zhang W, et al. Prevalence, risk factors, and clinical outcomes of remote intracerebral hemorrhage after intravenous thrombolysis in acute ischemic stroke: a systematic review and meta-analysis. J Neurol. 2023;270(2):651–61.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Internal Med. 1987;147(9):1561–4.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–110.
Honig A, Percy J, Sepehry AA, et al. Hemorrhagic transformation in acute ischemic stroke: a quantitative systematic review. J Clin Med. 2022;11(5):1162.
Rizos T, Horstmann S, Dittgen F, et al. Preexisting heart disease underlies newly diagnosed atrial fibrillation after acute ischemic stroke. Stroke. 2016;47(2):336–41.
Sposato LA, Cerasuolo JO, Cipriano LE, et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology. 2018;90(11):e924–31.
Yang XM, Rao ZZ, Gu HQ, et al. Atrial fibrillation known before or detected after stroke share similar risk of ischemic stroke recurrence and death. Stroke. 2019;50(5):1124–9.
Prats-Sánchez L, Camps-Renom P, Sotoca-Fernández J, et al. Remote intracerebral hemorrhage after intravenous thrombolysis: results from a multicenter study. Stroke. 2016;47(8):2003–9.
Zhong W, Yan S, Chen Z, et al. Stroke outcome of early antiplatelet in post-thrombolysis haemorrhagic infarction. J Neurol, Neurosurg Psychiatry. 2022;93:816–21.
Chen Y, Gong X, Zhong W, et al. Evaluation of a multilevel program to improve clinician adherence to management guidelines for acute ischemic stroke. JAMA Netw Open. 2022;5(5): e2210596.
The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28(11):2109–18.
Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37(2):556–61.
Tu HT, Campbell BC, Christensen S, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10(4):534–40.
Naess H, Waje-Andreassen U, Thomassen L. Persistent atrial fibrillation is associated with worse prognosis than paroxysmal atrial fibrillation in acute cerebral infarction. ISRN Cardiol. 2012;2012: 650915.
Koh YH, Lew LZW, Franke KB, et al. Predictive role of atrial fibrillation in cognitive decline: a systematic review and meta-analysis of 2.8 million individuals. Europace. 2022;24(8):1229–39.
Charidimou A, Turc G, Oppenheim C, et al. Microbleeds, cerebral hemorrhage, and functional outcome after stroke thrombolysis. Stroke. 2017;48(8):2084–90.
Chen Y, Yan S, Xu M, et al. More extensive white matter hyperintensity is linked with higher risk of remote intracerebral hemorrhage after intravenous thrombolysis. Eur J Neurol. 2018;25(2):380-e15.
Martí-Fàbregas J, Delgado-Mederos R, Granell E, et al. Microbleed burden and hematoma expansion in acute intracerebral hemorrhage. Eur Neurol. 2013;70(3–4):175–8.
Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501.
Cavallari I, Patti G. Early risk of mortality, cardiovascular events, and bleeding in patients with newly diagnosed atrial fibrillation. Eur Heart J Suppl. 2020;22(Suppl L):L110-l3.
Bassand JP, Virdone S, Goldhaber SZ, et al. Early risks of death, stroke/systemic embolism, and major bleeding in patients with newly diagnosed atrial fibrillation. Circulation. 2019;139(6):787–98.
Cappellari M, Moretto G, Bovi P. Repeated intravenous thrombolysis after recurrent stroke. A case series and review of the literature. J Neurol Sci. 2014;345(1–2):181–3.
Laible M, Jenetzky E, Möhlenbruch M, et al. Repeated intravenous treatment with recombinant tissue-type plasminogen activator in patients with acute ischemic stroke. Eur Neurol. 2015;74(3–4):127–34.
Kimura K, Aoki J, Shibazaki K, et al. New appearance of extraischemic microbleeds on T2*-weighted magnetic resonance imaging 24 hours after tissue-type plasminogen activator administration. Stroke. 2013;44(10):2776–81.
Miwa K, Koga M, Inoue M, et al. Cerebral microbleeds development after stroke thrombolysis: a secondary analysis of the THAWS randomized clinical trial. Int J Stroke. 2022;17(6):628–36.
Prats-Sanchez L, Martínez-Domeño A, Camps-Renom P, et al. Risk factors are different for deep and lobar remote hemorrhages after intravenous thrombolysis. PLoS ONE. 2017;12(6):e0178284.
Bernardo-Castro S, Sousa JA, Brás A, et al. Pathophysiology of blood–brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol. 2020;11:594672.
Acknowledgements
We thank all participating hospitals, their physicians and nurses.
Medical Writing/Editorial Assistance
The authors thank Research Square AJE LLC (https://www.aje.cn/) for English language editing, which was funded by the authors.
Funding
This study was supported by the National Natural Science Foundation of China (81971101, 82171276), the Science Technology Department of Zhejiang Province (2018C04011), and the editorial assistance of this manuscript and the rapid service fee were funded by the Science and Technology Plan of Jinhua City (2020-3-026, 2021-3-076).
Author information
Authors and Affiliations
Contributions
Zhicai Chen: Review and editing (lead), Conceptualization (equal); Hongfang Chen: Review and editing (equal), Funding acquisition (equal); Xiaoling Pan: Conceptualization (lead), Writing – original draft (lead), Investigation (equal), Methodology (lead), Data Curation (equal), Funding acquisition (equal); Yingjian Pei: Data Curation (lead), Methodology (equal), Writing – original draft (equal); Meixia Zhang: Data curation (equal), Methodology (equal), Investigation (equal); Wansi Zhong: methodology (equal), Investigation (equal); Jin Hu: Investigation (equal), Writing-review & editing (equal); Zhimin Wang: Investigation (equal), Writing-review & editing (equal); Dongjuan Xu: Investigation (equal), Writing-review & editing (equal); Min Lou: Writing-review & editing (equal), Funding acquisition (equal).
Corresponding authors
Ethics declarations
Conflict of Interest
Xiaoling Pan, Yingjian Pei, Meixia Zhang, Wansi Zhong, Jin Hu, Zhimin Wang, Dongjuan Xu, Min Lou, Hongfang Chen, and Zhicai Chen declare that they have no competing interests.
Ethical Approval
This study received approval from the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (2016 Annual Ethics Review No. 074). Meanwhile, this study was also approved by the local human ethics committee. The clinical investigation was conducted according to the principle expressed in the Declaration of Helsinki 1964. Written informed consent for IVT was obtained from all of the patients. Because patient information in the CASE-II was de-identified and anonymized before being released to the researchers, the informed consent requirement for this study was waived by the institutional review board.
Additional information
Prior Presentation: https://doi.org/10.1101/2023.02.22.23286328.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pan, X., Pei, Y., Zhang, M. et al. Association of Atrial Fibrillation with Remote Intracerebral Hemorrhage After Intravenous Thrombolysis: Results from a Multicenter Study in China. Neurol Ther 13, 127–139 (2024). https://doi.org/10.1007/s40120-023-00563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00563-9