Abstract
Introduction
Propofol is a general anesthetic option for deep brain stimulation (DBS) of the subthalamic nucleus (STN) of patients with Parkinson's disease (PD). However, its effects on STN activity and neuropsychological outcomes are controversial. The optimal propofol anesthesia for asleep DBS is unknown. This study investigated the safety and effectiveness of an optimized propofol anesthesia regimen in asleep DBS.
Methods
This retrospective study enrolled 68 PD patients undergoing bilateral STN-DBS surgery. All patients received local scalp anesthesia, with (asleep group, n = 35) or without (awake group, n = 33) propofol-remifentanil general anesthesia by target-controlled infusion under electroencephalogram monitoring. The primary outcome was subthalamic neuronal spiking characterization during microelectrode recording. The secondary outcomes were clinical outcomes including motor, cognition, mind, sleep, and quality of life at 6 months.
Results
Significantly increased delta and theta power were obtained under propofol anesthesia (awake vs. asleep group, mean ± standard deviation; delta: 31.97 ± 9.87 vs. 39.77 ± 10.56, p < 0.01; theta: 21.09 ± 5.55 vs. 24.82 ± 6.63, p = 0.01). After excluding the influence of confounding factors of age and preoperative motor scores, there was a statistically significant influence on the delta, theta, and alpha power of STN neuronal activity under different anesthesia regimens (delta: β = 2.64, p < 0.01; theta: β = 2.11, p < 0.01; alpha: β = 1.42, p = 0.01). There were no differences in modified burst index, firing rate, tract numbers of microelectrode recording, and other clinical outcomes between the two groups.
Conclusion
Optimized propofol anesthesia enhanced the delta, theta, and alpha power in STN compared with the awake technique and likely contributed to target recognition under propofol anesthesia. These results demonstrate that propofol is suitable, but needs to be optimized, for asleep STN-DBS.
Trial Registration
Chinese Clinical Trial Registry Identification number: ChiCTR2100045942. Registered 29 April 2021–Retrospectively registered
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Propofol is a general anesthetic option for deep brain stimulation (DBS) of the subthalamic nucleus (STN) of patients with Parkinson's disease (PD). However, its effects on STN activity and neuropsychological outcomes are controversial. |
This study investigated the safety and effectiveness of an optimized propofol anesthesia regimen in asleep DBS. |
What was learned from the study? |
Optimized propofol anesthesia enhanced the delta, theta, and alpha power in STN compared with the awake technique, and there were no differences in clinical outcomes between the two groups. |
These results demonstrate that propofol is suitable, but needs to be optimized, for asleep STN-DBS. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14637549.
Introduction
Bilateral subthalamic nucleus (STN) deep brain stimulation (DBS) is an effective treatment for advanced Parkinson's disease (PD) [1]. The application of intraoperative microelectrode recording (MER) is conducive to target definition but traditionally requires an awake and cooperative patient [2]. Some patients with PD must undergo STN-DBS surgery under an asleep condition due to severe anxiety, rigidity, and dystonia in the off-drug phase. Several DBS centers always conduct surgery under general anesthesia. Different general anesthetic methods are used in different DBS centers, such as inhalation anesthesia, sedation with dexmedetomidine, or propofol-based intravenous anesthesia [3,4,5,6,7], but none of these has proven to be superior [8]. The optimal anesthetic regimen should provide sufficient comfort and safety, but should not affect the intraoperative electrophysiological signal and clinical efficacy [8,9,10,11].
Propofol is a widely used intravenous anesthetic agent with the advantages of rapid metabolism, safety, controllable anesthetic state, and neuroprotective effects [8, 12,13,14]. However, its effects on STN activity and neuropsychological outcomes on asleep DBS for PD remain controversial. Some studies have reported STN activity interference with propofol anesthesia, such as the absence of typical widening of the background noise baseline [15] and a decrease in the root mean square [16] and the relative beta power [17]. In contrast, others have reported successful propofol use by combining anesthesia depth monitoring [18, 19], target-controlled infusion (TCI) techniques [18], and a dosage reduction [18, 20] during MER under propofol. These studies suggest that the contradictory results could be due to the various concentration, dose, mode, and timing of propofol administration [17, 21, 22]. Therefore, it is necessary to optimize propofol anesthesia for asleep DBS surgery.
There are limited data on the effectiveness of propofol anesthesia in postoperative improvement of non-motor symptoms including cognition, mental status, sleep, and quality of life in PD. Two studies have reported adverse effects on postoperative neuropsychological outcomes and memory function after DBS under propofol anesthesia [15, 23]. As significant cognitive decline has also been observed under volatile anesthesia [24], we hypothesized that the possible reason for the adverse effects is related to target location and postoperative stimulation parameters, rather than propofol. Therefore, establishing the effectiveness of propofol anesthesia in asleep DBS requires further analysis.
In this study, we used an electroencephalogram monitoring-guided propofol-remifentanil TCI with a multimodal analgesic regimen for asleep STN-DBS procedures. We quantitatively and qualitatively analyzed the neuronal signatures, including power distribution, oscillatory activity, total numbers of MER trajectory, and length of STN. The neuronal activity of STN and clinical outcomes in asleep PD patients were compared with those in age- and disease-matched awake patients. The goal of the study was to verify the safety and effectiveness of an optimized propofol general anesthesia regimen. In particular, we hypothesized that propofol anesthesia should be further optimized and widely used in asleep DBS because it has no deleterious effects on electrophysiological signals and clinical outcomes.
Methods
Patient Population
We performed a retrospective offline analysis of MER data of 159 consecutive PD patients undergoing bilateral STN-DBS surgery in the First Affiliated Hospital of Sun Yat-sen University from July 2008 to August 2019. This manuscript adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines. The study was approved by the relevant local ICE for clinical research and animal trials of the First Affiliated Hospital of Sun Yat-sen University (reference numbers IIT2016192). Because this study retrospectively analyzed routine clinical data, written informed consent from the patients was not required. The retrospective study was grouped according to the type of anesthesia used. The method of anesthesia was determined by the patients according to their own wishes. All anesthesia in this study was performed by the same anesthesiologist. Because some conditions seriously affect the results or analyses, we exclude data for the following cases: (1) patients who had undergone brain surgery before surgery, (2) patients who took special drugs (antidepressants, benzodiazepines, or antiparkinsonian medications) that would affect the MER data before surgery, (3) the regimen of anesthesia was changed during the MER (for example, the awake patients changed their will for various reasons and then received general anesthesia, or asleep patients received other sedative drugs such as sevoflurane), (4) MER data were lost or incomplete, and (5) the quality of the MER data was poor. Therefore, we finally analyzed MER data of 68 PD patients. Figure 1 shows the flowchart. Of the remaining 68 patients, 33 patients were included in the awake group and 35 patients were included in the asleep group, depending on the method of anesthesia they received.
Imaging and Stereotactic Procedures
All antiparkinsonian medications were withdrawn at 12 h before the surgery. The head of each patient was scanned using a 3T magnetic resonance imaging (MRI) machine to identify the STN. On the day of the surgery, the patient was positioned in a stereotactic frame (Leksell G type) under local anesthesia to perform computed tomography (CT) scanning, and the MRI and CT images were then merged. The VectorVision neuronavigational system was used to calculate the three-dimensional coordinates of the STN and plan out 10 electrode tracks.
Anesthetic Technique
All patients received local scalp anesthesia with ropivacaine 0.5%. In the awake group, 33 patients were conscious during MER and received electrode implantation without sedatives. In the asleep group, 35 patients underwent endotracheal intubation under total intravenous general anesthesia. Dexmedetomidine was infused (0.3 μg/kg) before induction. For induction, propofol was administered by TCI (Alaris Medical Systems, Inc., San Diego, CA, USA) based on the Marsh model with a target plasma concentration of 2 μg/ml. Sufentanil (0.2 μg/kg) and cisatracurium (0.2 mg/kg) were given intravenously. After endotracheal intubation, anesthesia was maintained with propofol TCI (1–2 μg/ml), remifentanil TCI (4–6 ng/ml), and cisatracurium (1.5 μg/kg/min). For TCI remifentanil, a target plasma concentration based on the Minto model was maintained by a TCI pump (Orchestra® Base Primea, Fresenius Vial, Brezins, France). At least 10 min before MER, the target propofol and remifentanil concentrations were reduced to 1–2 μg/ml and 2–3 ng/ml, respectively. During MER, the anesthetic depth was titrated to maintain it at the C level on Narcotrend monitoring (MonitorTechnik, Bad Bramstedt, Germany).
Surgical Procedures and Intraoperative MER
All DBS surgical operations were performed by the same surgeon. After scalp incision and perforation, a single-channel microelectrode with a diameter of 25 μm was conducted by a manual microdrive device (microTargeting®, FHC Inc., Bowdoin, ME, USA). MER started 15 mm above the theoretical STN target at increments of 0.5 mm for 10 s at each depth until the typical neuronal discharges of the STN were no longer observed. The entrance and exit of STN were mainly based on visual and auditory evaluation. Typically, the background activity, noise, and peak frequency of spontaneous neurons apparently increased when the microelectrode entered STN and decreased when it was out of STN [25]. A surgical monitoring system (Leadpoint®, Medtronic, Englewood, CO, USA) was used to record and store MER data. All data were filtered using a 500-Hz high-pass filter and a 5-kHz low-pass filter with a sampling rate of 24 kHz. The path length of the microelectrode through the entire STN was recorded as the length of the STN. The criteria for selecting the optimal trajectory were based on Benazzouz et al. [25]. Both groups of patients were tested for passive movement of the contralateral extremities.
Permanent quadripolar DBS electrodes (DBS model 3389 electrode, Medtronic, Inc., Minneapolis, MN, USA) were implanted along the optimal trajectory after X-ray examination. The stereotactic frame was removed, and infraclavicular placement of the internal pulse generators (Medtronic, Minneapolis, MN) was performed under general anesthesia. The pulse generator was turned on 1 month after the operation.
Extraction of Spiking Activity in the STN and Parameter Calculations
Data Screening
MER signals recorded along the DBS electrode tracks were collected for off-line analysis. In this study, the microelectrode was inserted into the STN in increments of 0.5 mm, and the mean length of the electrophysiological signal of the unilateral STN was 6 mm. To reduce errors caused by differences in data-depth ranges, the boundary fragments were removed and the middle 4 mm section was selected for further analysis. Therefore, analyses were conducted on the middle 8 fragments for each STN. Collectively, a total of 1088 groups of MER data were contained [68 subjects × 8 depths × 2(bilateral)].
Based on extraction of all spikes from raw MER signals, according to the eight different depth datasets of each side of the STN of each patient, the firing frequency of the bilateral STN of each patient was calculated. Specifically, 0–100 Hz was selected as the main frequency band for signal analysis. This main frequency band was further subdivided into five frequency bands: delta, theta, alpha, beta, and gamma. The relationships among the mean spike power in different frequency bands under different anesthetic methods and different sides of the STN were then determined.
Action Potential (Spike) Extraction
The action-potential spike reflects the firing characteristics of the STN. According to the spikes extracted from the raw MER signals, the modified burst index (MBI) and firing rate (FR) of the spikes were calculated to reflect the characteristics of STN firing in order to analyze the effects of different anesthetic methods on the firing characteristics of the STN. To extract the envelope of MER data, the Hilbert transform is used, which is the convolution result of the original signal x(t) and the signal 1/πt [26]:
To estimate the envelope of spikes, the modulus of the analytic signal is calculated as \(\left| {\tilde{x}\left( t \right)} \right| = \sqrt {x\left( t \right)^{2} + \hat{x}\left( t \right)^{2} }\).
The autocorrelogram is calculated based on the detected spike sequence as [27]:
where \(\rho \left( t \right)\) is the spike sequence, \(T\) indicates the data length, \(r = \frac{n}{T}\) is the firing rate of the spike, and \(n\) is the number of spikes in the time \(T\).
The raw MER signals contained both background noise and spikes. To extract spikes, background noise was filtered out. The background noise in the STN MER was mainly composed of microelectrode-recording equipment noise, biological background noise, and action potentials generated by neurons surrounding the target neurons during the acquisition process. Most of these noises were Gaussian noises, and their standard deviation can be calculated as the median of the absolute values of the raw signals divided by 0.6745: [28]
where s(t) represents the raw MER signal. The threshold (Th) of the spikes in the signals is four times the standard deviation of Gaussian noise, i.e., Th = 4σ [21].
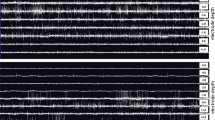
Figure 2 shows the process of spike extraction from one typical signal with a length of 1 s. Figure 2A shows the raw MER signal, and Fig. 2B shows the MER signal with detected spikes. The threshold of this signal was calculated, and data with an amplitude higher than the threshold were considered as spikes. The times corresponding to the selected spikes were assigned a pulse value of 1 and the rest positions were assigned an amplitude of 0 to construct a new spike signal sequence (spike train), as shown in Fig. 2C.
The MBI is defined as the ratio of the number of inter-spike intervals (ISI) less than 10 ms to the number of ISIs greater than 10 ms [21] which reflects the characteristics of the timing of STN. The equation for this calculation is as follows:
where Nl represents the number of ISIs shorter than 10 ms, and Ng represents the number of ISIs greater than 10 ms.
FR is defined as the number of spikes in 1 s [21]. The equation for this calculation is as follows:
where tspk represents the time sequence in which the spikes appear in the STN MER signals, and T represents the duration of the signals in seconds.
The means of the overall MBIs and FRs in the asleep and awake groups were calculated according to the multiple sets of MER signals from the bilateral STNs of the included patients. The equation is as follows:
where xi represents the MBI or the FR value of the ith patient and n represents the total number of patients.
The standard deviations of the overall MBIs and FRs in the awake and asleep group were calculated according to the MBI and FR data from the bilateral STN in each patient, which were used to measure the degree of data dispersion.
Clinical Outcomes
Motor and non-motor evaluations were performed by professionally trained neurologists before surgery and 6 months following surgery. Motor symptoms were evaluated using the third part of the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS III). The improvement rate of motor symptoms is the percentage of the difference of scores before and after surgery divided by the score before surgery. Non-motor assessments included assessments of cognition, sleep, quality of life, and mental status. The 39-item Parkinson’s Disease Questionnaire (PDQ39) and Non-Motor Symptom Scale (NMSS) were used to assess each patient’s quality of life. The Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) were used for mental assessments. The Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), and Parkinson's Disease Sleep Scale (PDSS) were used to evaluate sleep quality and the severity of sleep disorders. We used the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) to assess the level of cognitive function in each patient.
In all cases, the common postoperative adverse events were recorded, and these were as follows: (1) complications related to surgery or anesthesia, such as incision-related infection, pneumonia, deep vein thrombosis, air embolism, pulmonary edema, hemiplegia, and death; (2) complications related to hardware, such as intracranial hematoma, implant infection, and electrode displacement/damage; (3) complications related to stimulation, such as difficulty opening eyes, dysarthria, and dysphagia; (4) other complications, such as neuropsychiatric symptoms, drowsiness, restlessness, delirium, and indifference.
Statistical Analysis
SPSS version 26.0 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD). Categorical variables were expressed as number (percentage).
The mean spike power in the five frequency bands of each sample group was compared using an independent-samples t test. Two-way analysis of variance (ANOVA) was used to determine whether the average frequencies of different levels of spikes of the two factors (different anesthetic methods and different sides of STN) were significantly different between the two groups, as well as whether there was an interaction between the average spike frequency of the two factors. To account for variance of age and MDS-UPDRS III between the two groups, we conducted a linear regression analysis to regress out the effects of age and preoperative MDS-UPDRS III on the power of STN neuronal activity. Similarly, two-way ANOVA was used to determine whether there was no interaction between the MBIs and FRs of different anesthetic methods and different sides of the STN.
Differences in the postoperative improvements in ESS, PDQ-39, and MOCA, MDS-UPDRS III, PSQI, PDSS, NMSS, HAMD, HAMA, and MMSE scores between the awake and asleep group were tested using an independent-samples t test. Categorical variables were compared using Pearson’s chi-square test. Significance was recognized when p < 0.05.
Results
Baseline Characteristics
Baseline characteristics of the 68 analyzed PD patients are listed in Table 1. The mean age was 56.35 ± 9.09 years. Forty-three (63.2%) of the patients were male. The mean PD duration was 9.43 ± 2.98 years. The levodopa equivalent daily dose was calculated according to drug correspondence proposed by Tomlinson et al. [29]. The baseline values of the two groups were comparable (all p values were greater than 0.05).
Spectral Analysis
During MER, well-recognized STN signals recorded during propofol anesthesia were similar to those detected under the awake condition (Fig. 3). In the awake group, there was no difference in the mean spike power in the delta, theta, alpha, beta, and gamma between the left and right STN (Fig. 4A). In the asleep group, there was also no difference in the mean spike power between the left and right STN (Fig. 4B). For left STN, the mean spike power was significantly different between the two groups in the theta band (awake vs. asleep group, mean ± SD; theta: 20.99 ± 5.45 vs. 25.25 ± 8.97, p = 0.02, see Table S1 and Table S2 in the supplementary material for details), while there were no differences in the delta, alpha, beta, and gamma frequency bands (Fig. 4C). For right STN, the mean spike power was significantly different between the two groups in the delta band (awake vs. asleep group, mean ± SD; delta: 31.11 ± 12.57 vs. 40.61 ± 13.85, p < 0.01; Fig. 4D, see Table S1 and Table S2 in the supplementary material for details). For bilateral STN, the mean spike power between the two groups was significantly different in the delta and theta bands (awake vs. asleep group, mean ± SD; delta: 31.97 ± 9.87 vs. 39.77 ± 10.56, p < 0.01; theta: 21.09 ± 5.55 vs. 24.82 ± 6.63, p = 0.01; Fig. 5, see Tables S1 and S2 in the supplementary material for details). The results analyzed using two-way ANOVA and independent-samples t tests were consistent (see Table S2 in the supplementary material for details). There was no interaction between the average spike power across different anesthetic methods and different sides of the STN. Linear regression analysis showed that there was a statistically significant influence on the delta, theta, and alpha power of STN neuronal activity under different anesthesia regimens (delta: β = 2.64, p < 0.01; theta: β = 2.11, p < 0.01; alpha: β = 1.42, p = 0.01, see Table 2).
MER Comparisons between the awake and asleep groups. A, B MER raw data: one representative example of STN MER data in 1 s in the awake group and asleep group, respectively. C, D Envelope waveform: computed from the Hilbert transformed MER signal within the marked area of (A, B, respectively). E, F The autocorrelogram of the 10-s MER raw data in the awake group and asleep group. MER: microelectrode recording; STN: subthalamic nucleus
The power spectrum: the mean and SD between 0 and 100 Hz for the two groups. A Violin plots illustrating differences in the mean spike power in the delta, theta, alpha, beta, and gamma between the left and right STN in the awake group. B Differences in the mean spike power in the delta, theta, alpha, beta, and gamma between the left and right STN in the asleep group. C For left STN, the differences mean spike power between the two groups. There was a significant difference in the theta band for the left STN between the two groups. D For right STN, the mean spike power differences between the two groups. There was a significant difference in the theta band for the left STN between the two groups. Significant changes are highlighted with a * (p < 0.05). STN: subthalamic nucleus.
Difference in power spectrum between the awake and asleep groups. The red and blue lines are the mean spectrum of the MER in the awake and asleep groups, respectively. Significant differences are highlighted in green (p < 0.05). The powers of delta- and theta-frequency oscillation in the asleep group were significantly increased than those in the awake group (p < 0.01 p = 0.01). MER: microelectrode recording
Comparisons of MBIs and FRs
There was no significant difference between the MBIs and FRs of the STN on different sides or on the same side of the brain within each patient (all p > 0.05; see Table S3 in the supplementary material for details). There was no interaction between the MBIs and FRs of different anesthetic methods and different sides of the STN (see Table S4 in the supplementary material for details).
Comparisons of MER Trajectories and Length of STN
The operation results of the two groups were compared (see Table S5 in the supplementary material for details). The mean total number (bilateral STN) of MER trajectories was 3.12 ± 1.54. There was no statistical difference between the two groups in terms of the total number of MER trajectories, the ratio when the total number of MER trajectories was equal to 2, and the length of the left STN (p > 0.05, for all). Although the length of the right STN in the asleep group was statistically longer than that in the awake group (p = 0.02), there was limited clinical relevance.
Comparisons of Clinical Outcomes at the 6-Month Follow-up
Table 3 compares the improvement in motor symptoms and non-motor symptoms between the two groups. There was no significant difference between the two groups (all p > 0.05). In the adverse events category, no patient suffered permanent deficits, bleeding, or death. In the awake group, a 6-month follow-up result of adverse events showed that one case had an incision-induced infection. One patient developed mental abnormality as a result of self-administered overdoses of antiparkinsonian medications. In the asleep group, there were no occurrences of intraoperative awareness, perioperative neurocognitive disorders, or mental disorders. Five cases had incision-related infections and seven cases developed transient hiccups. One patient had drug-induced hallucinations, with a previous history of this before surgery. One patient with respiratory comorbid conditions experienced dyspnea after tracheal extubation failure and required re-intubation.
Discussion
In this study, the anesthetic approach of Narcotrend-optimized anesthesia-depth control and TCI-guided propofol and remifentanil dosage achieved good STN signals and clinical efficacy. This regimen allowed effective MER under propofol without affecting the bursting or firing rates, and increased the power of delta and theta in the MERs for the asleep group. We found no significant changes in the debated non-motor effects between two groups, in particular cognitive and psychobehavioral.
Previous studies have shown that the use of remifentanil or propofol inhibits MER signals, which seems to be different from our results [16, 30, 31]. However, it is worth noting that Mciver et al. [30] used a bolus administration (propofol 0.3 mg/kg or remifentanil 0.05 mg/kg) and Benady et al. [31] used continuous constant rate administration (propofol 50 μg/kg/min or remifentanil 0.1 μg/kg/min) without an objective level of consciousness assessment or local scalp anesthesia. In this study, we used an electroencephalogram monitoring-guided propofol-remifentanil TCI (1–2 μg/ml and 2–3 ng/ml, respectively) with the local scalp anesthesia regimen. Typically, continuous infusion of 0.1–0.5 ug/kg/min remifentanil is approximately 3–7 ng/ml when using a TCI [32]. Therefore, our doses tended to be lower. At the same time, it is important to note that our study did not explore the effect of a single drug on MER. These controversial results suggest the importance of choice, dosage, and the timing of anesthetic agents for the recording of STN neuron firing [15,16,17, 21, 33]. Our combined use of propofol and remifentanil has the advantage that these drugs exhibit synergistic effects, which reduces their required doses and any associated adverse drug reactions. Also, a multimodal analgesic regimen, including local scalp anesthesia, and a small dose of dexmedetomidine and flurbiprofen can reduce the amount of analgesics. For PD patients, lower-than-average propofol doses are sufficient [9, 34]. Previous research showed that the propofol requirement for induction and remifentanil requirement for tracheal intubation and skin incision were significantly reduced compared to the requirements for non-PD patients [35, 36]. This phenomenon may be due to increased sensitivity to anesthetics in PD patients due to degenerative brain lesions, resulting in abnormal pharmacodynamic characteristics of the anesthetics. Therefore, the conventional pharmacodynamic concept and drug infusion methods of administering IV drugs (bolus dose and continuous infusion) might lead to a relative overdose of propofol and opioid analgesics during MER. This could further affect the recording of STN neuron firing and lead to compromise of cardiovascular function, delayed emergence, and postoperative delirium due to over-sedation. Compared to traditional drug infusion methods, TCI may be more suitable for patients with PD especially during MER, with the advantage of achieving micro-injection and more precisely controlled target drug concentration in the plasma or at the effect site. On the other hand, monitoring the depth of anesthesia may be sufficiently reliable to maintain proper light-state anesthesia during MER under a low dose of narcotics. This has significance in minimizing the interference of electrophysiological signals, preventing intraoperative consciousness and intracranial hypertension caused by physical movement and asphyxia. It will help to avoid hemodynamic events caused by deep anesthesia and postoperative cognitive dysfunction.
A similar study demonstrated the feasibility of using propofol and remifentanil combined with BIS monitoring for modulating anesthesia during STN-DBS surgery, with propofol TCI and continuous infusion of remifentanil [15]. However, this study did not perform quantitative analysis of intraoperative electrophysiology and did not follow up on the outcomes of postoperative non-motor symptoms (such as cognitive/mental function). MER can be achieved during asleep DBS as long as the drug and the depth of anesthesia are appropriate, but it is also important for anesthesiologists to protect the cognitive/mental aspects of elderly PD patients [18]. Therefore, emphasis should be placed on the choice of anesthetic drugs used. Compared with sevoflurane, propofol anesthesia can reduce the incidence of postoperative delirium in elderly patients, and it has the advantage of protecting the fragile brains of the elderly. Additionally, propofol anesthesia can reduce the incidence of delayed neurocognitive recovery in elderly patients after tumor surgeries [12]. In a vulnerable mouse transgenic model, the incidence of cognitive impairment and neuroinflammatory sequelae after using propofol were lower than after using inhaled anesthetic drugs [37]. It has been reported that propofol can alleviate 1-methyl-4-phenylpyridinium-induced oxidative stress by increasing the content of reactive oxygen and ATP levels, as well as by improving mitochondrial membrane potential. This prevents the neurotoxicity of 1-methyl-4-benzene pyridinium that leads to PD [14]. In general, propofol is more suitable for anesthesia in elderly patients.
Propofol induces variable anesthetic effects when it is used at different doses and acts on different receptors. However, it is still unclear which molecular targets have the greatest clinical effects and which cause unnecessary side effects. Different concentrations of propofol have different effects on the central nervous system and electroencephalogram. At sedative concentrations, propofol significantly decreased the activity of neurons in the cortical neural network, weakened alpha power, and enhanced frontal beta power. At higher hypnotic concentrations, the activity of neurons in the subcortical structures was also affected, while the slow-wave activity in the delta band became more prominent and the frontal beta power decreased [38]. It seems that the effect of propofol on the basal ganglia discharge is closely related to the dose. For patients with dystonia, the average firing rates of internal globus pallidus were lower under propofol anesthesia [39]. Similarly, it appears to be dose-related, as one of the patients receiving a low dose of propofol (60 mg IV) had firing patterns that were not different from those in other non-sedated patients. Our data show that propofol anesthesia does not inhibit the MER signal, but it does enhance the power of low-frequency signals. This finding, which was not observed in the awake group, may be used as an electrophysiological marker for STN signals during MER under propofol anesthesia and helps to target localization. It supports the view that propofol anesthesia needs to optimize the depth of anesthesia and increase the controllability of anesthesia from the perspective of concentration, dose, and time of administration. No studies have determined whether increased low-frequency power during anesthesia directly affects clinical prognostic outcomes. However, the current hypothesis of the mechanism of STN-DBS, "information damage" and "synaptic filtering" [40], indicates that the lack of low-frequency information increases the limitation of DBS, and propofol enhancement of low-frequency information during anesthesia may play a short-term role by compensating for this defect.
This study had some limitations. The anesthesia method was determined by patient’s choice so patients were not randomly assigned to the awake group and asleep group. Nevertheless, the comparable baseline for both groups and the rigorous surgery, anesthesia, and follow-up procedures performed by the same team strengthen the results. In this study, spike sorting was not performed when comparing the FRs, MBIs, and total power of the two groups. Although some related studies were also based on data without spike sorting, our results are not sufficient to reach any conclusions at the level of single units. Multi-center large sample-size prospective studies are required to distinguish the subtle differences in electrophysiological characteristics and long-term clinical outcomes between the two groups that we reported here.
Conclusions
We conclude that the anesthetic regimen reported here, during STN-DBS, is safe and effective for patients with advanced PD. Propofol anesthesia in asleep DBS needs to optimize the depth of anesthesia and increase the controllability of anesthesia from the aspects of concentration, dose, and time of administration. The increased delta, theta, and alpha power observed during MER reflects characteristic signature in the STN under optimized propofol anesthesia, which may be helpful for target identification.
References
Castrioto A, Lhommee E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13:287–305.
Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81.
Lin SH, Chen TY, Lin SZ, et al. Subthalamic deep brain stimulation after anesthetic inhalation in Parkinson disease: a preliminary study. J Neurosurg. 2008;109:238–44.
Tsai ST, Tseng GF, Kuo CC, Chen TY, Chen SY. Sevoflurane and Parkinson’s disease: subthalamic nucleus neuronal activity and clinical outcome of deep brain stimulation. Anesthesiology. 2020;132:1034–44.
Sassi M, Zekaj E, Grotta A, et al. Safety in the use of dexmedetomidine (precedex) for deep brain stimulation surgery: our experience in 23 randomized patients. Neuromodulation. 2013;16:401–6.
Fluchere F, Witjas T, Eusebio A, et al. Controlled general anaesthesia for subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1167–73.
Harries AM, Kausar J, Roberts SAG, et al. Deep brain stimulation of the subthalamic nucleus for advanced Parkinson disease using general anesthesia: long-term results. J Neurosurg. 2012;116:107–13.
Poon CC, Irwin MG. Anaesthesia for deep brain stimulation and in patients with implanted neurostimulator devices. Br J Anaesth. 2009;103:152–65.
Grant R, Gruenbaum S, Gerrard J. Anaesthesia for deep brain stimulation: a review. Curr Opin Anaesthesiol. 2015;28:505–10.
Lange M, Zech N, Seemann M, et al. Anesthesiologic regimen and intraoperative delirium in deep brain stimulation surgery for Parkinson’s disease. J Neurol Sci. 2015;355:168–73.
Venkatraghavan L, Luciano M, Manninen P. Review article: anesthetic management of patients undergoing deep brain stimulator insertion. Anesth Analg. 2010;110:1138–45.
Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121:595–604.
Vasileiou I, Xanthos T, Koudouna E, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8.
Wang S, Song T, Leng C, Lan K, Ning J, Chu H. Propofol protects against the neurotoxicity of 1-methyl-4-phenylpyridinium. Mol Med Rep. 2016;13:309–14.
Hertel F, Zuchner M, Weimar I, et al. Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson’s disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery. 2006;59:E1138.
Raz A, Eimerl D, Zaidel A, Bergman H, Israel Z. Propofol decreases neuronal population spiking activity in the subthalamic nucleus of Parkinsonian patients. Anesth Analg. 2010;111:1285–9.
Martinez-Simon A, Alegre M, Honorato-Cia C, et al. Effect of dexmedetomidine and propofol on basal ganglia activity in Parkinson disease: a controlled clinical trial. Anesthesiology. 2017;126:1033–42.
Lefaucheur JP, Gurruchaga JM, Pollin B, et al. Outcome of bilateral subthalamic nucleus stimulation in the treatment of Parkinson’s disease: correlation with intra-operative multi-unit recordings but not with the type of anaesthesia. Eur Neurol. 2008;60:186–99.
Duque P, Mateo O, Ruiz F, de Viloria J, Contreras A, Grandas F. Intraoperative microrecording under general anaesthesia with bispectral analysis monitoring in a case of deep brain stimulation surgery for Parkinson’s disease. Eur J Neurol. 2008;15:e76–7.
Kocabicak E, Aygun D, Alptekin O, et al. Conversion of local anesthesia-guided deep brain stimulation of the subthalamic nucleus to general anesthesia. J Neurol Surg A Cent Eur Neurosurg. 2013;74:332–4.
Lin SH, Lai HY, Lo YC, et al. Decreased power but preserved bursting features of subthalamic neuronal signals in advanced Parkinson’s patients under controlled desflurane inhalation anesthesia. Front Neurosci. 2017;11:701.
Bos MJ, Buhre W, Temel Y, Joosten EAJ, Absalom AR, Janssen MLF. Effect of anesthesia on microelectrode recordings during deep brain stimulation surgery: a narrative review. J Neurosurg Anesthesiol. 2020. https://doi.org/10.1097/ANA.0000000000000673.
Tsai ST, Chen SY. Letter to the editor: deep brain stimulation and general anesthesia. J Neurosurg. 2012;117:1207–8.
Chen SY, Tsai ST, Lin SH, et al. Subthalamic deep brain stimulation in Parkinson’s disease under different anesthetic modalities: a comparative cohort study. Stereotact Funct Neurosurg. 2011;89:372–80.
Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17:S145–9.
Benitez D, Gaydecki PA, Zaidi A, Fitzpatrick AP. The use of the Hilbert transform in ECG signal analysis. Comput Biol Med. 2001;31:399–406.
Abbott LF, Dayan P. Theoretical neuroscience: computational and mathematical modeling of neural systems. Comput Neurosci. 2004;79:113.
Dolan K, Martens HC, Schuurman PR, Bour LJ. Automatic noise-level detection for extra-cellular micro-electrode recordings. Med Biol Eng Comput. 2009;47:791–800.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–53.
Maciver MB, Bronte-Stewart HM, Henderson JM, Jaffe RA, Brock-Utne JG. Human subthalamic neuron spiking exhibits subtle responses to sedatives. Anesthesiology. 2011;115:254–64.
Benady A, Zadik S, Eimerl D, et al. Sedative drugs modulate the neuronal activity in the subthalamic nucleus of parkinsonian patients. Sci Rep. 2020;10:14536.
Yu E, Tran D, Lam S, Irwin M. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. 2016;71:1347–62.
Krishna V, Elias G, Sammartino F, et al. The effect of dexmedetomidine on the firing properties of STN neurons in Parkinson’s disease. Eur J Neurosci. 2015;42:2070–7.
Fabregas N, Rapado J, Gambus PL, et al. Modeling of the sedative and airway obstruction effects of propofol in patients with Parkinson disease undergoing stereotactic surgery. Anesthesiology. 2002;97:1378–86.
Xu XP, Yu XY, Wu X, et al. Propofol requirement for induction of unconsciousness is reduced in patients with Parkinson’s disease: a case control study. Biomed Res Int. 2015;2015:1–5.
Wang JF, Xu XP, Yu XY, et al. Remifentanil requirement for inhibiting responses to tracheal intubation and skin incision is reduced in patients with Parkinson’s disease undergoing deep brain stimulator implantation. J Neurosurg Anesthesiol. 2016;28:303–8.
Deffains M, Bergman H. Parkinsonism-related beta oscillations in the primate basal ganglia networks: recent advances and clinical implications. Parkinsonism Relat Disord. 2019;59:2–8.
Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20.
Hutchison W, Lang A, Dostrovsky J, Lozano A. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol. 2003;53:480–8.
Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–60.
Acknowledgements
The authors wish to thank the patients for the use of their data in this study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Statistical Analysis and Other Assistance
Constructive and critical comments in the preparation of this article were provided by Shi-Ming Shi at the Department of Pediatric Surgery, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China. Statistical analysis in the preparation of this article was provided by Xiao-Ling Huang at the Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong, China.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors Contributions
Conception and design: Jin-Long Liu, Nan Jiang, Yu-Ting Ling. Acquisition of data: Jin-Long Liu, Nan Jiang, Yu-Ting Ling, Chao Yang, Li-Nan Zhang, Qian-Qian Guo, Xing-Yi Jin, Ling Chen, Zhi-Guo Zhang. Analysis and interpretation of data: Jin-Long Liu, Yu-Ting Ling, Wen-Biao Xian, Yi Liu, Xing-Yi Jin, Bin Wu, Chang-Ming Zhang, Ling Chen, Zhi-Guo Zhang. Drafting the article: Nan Jiang, Yu-Ting Ling, Xing-Yi Jin, Bin Wu, Chang-Ming Zhang, Zhi-Guo Zhang. Critically revising the article: Nan Jiang, Yu-Ting Ling, Wen-Biao Xian, Xing-Yi Jin, Bin Wu, Chang-Ming Zhang, Zhi-Guo Zhang. Reviewed submitted version of manuscript: Jin-Long Liu, Nan Jiang, Yu-Ting Ling, Bin Wu, Chang-Ming Zhang, Zhi-Guo Zhang. Approved the final version of the manuscript on behalf of all authors: Nan Jiang, Jin-Long Liu. Statistical analysis: Yu-Ting Ling, Xing-Yi Jin. Study supervision: Jin-Long Liu, Nan Jiang, Ling Chen, Zhi-Guo Zhang.
Disclosures
Nan Jiang, Yu-Ting Ling, Chao Yang, Yi Liu, Wen-Biao Xian, Li-Nan Zhang, Qian-Qian Guo, Xing-Yi Jin, Bin Wu, Chang-Ming Zhang, Ling Chen, Zhi-Guo Zhang, and Jin-Long Liu have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the relevant local ICE for clinical research and animal trials of the First Affiliated Hospital of Sun Yat-sen University (reference numbers IIT2016192). Informed consent was not required because this is a retrospective study. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jiang, N., Ling, YT., Yang, C. et al. Optimized Propofol Anesthesia Increases Power of Subthalamic Neuronal Activity in Patients with Parkinson’s Disease Undergoing Deep Brain Stimulation. Neurol Ther 10, 785–802 (2021). https://doi.org/10.1007/s40120-021-00259-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-021-00259-y