Abstract
Economic sustainability is of paramount importance in the rapidly evolving therapeutic scenario of multiple sclerosis (MS). Glatiramoids are a class of drugs whose forefather, glatiramer acetate, has been used as a disease modifying drug (DMD) in patients with MS for over 20 years. Its patent expired in 2015; new versions of such drug are nowadays available on the market, potentially contributing to lowering prices and enhancing a better allocation of economic resources. In this review, we analyze the recommendations underlying the approval of both generic drugs and biosimilars by regulatory authorities, and we provide methodological tools to contextualize the design of studies on these new classes of drugs. We examine in more detail the preclinical and clinical data of Copemyl®, a new member of the glatiramoid class, focusing on its biological and immunological properties and illustrating randomized controlled trials that led to its authorization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glatiramoids are a class of drugs consisting in a group of heterogenous polypeptide mixtures comprising four amino acids: l-glutamic acid, l-alanine, l-lysine, and l-tyrosine [1]. Glatiramer acetate (GA) is the forefather of this class [1] and is used as a disease modifying drug (DMD) in patients with multiple sclerosis (MS) [2]. It was originally conceived as a synthetic analogue of myelin basic protein, and initially (and surprisingly) tested to induce experimental autoimmune encephalomyelitis (EAE), the experimental model of MS in animals [3]. After it was shown that, instead of promoting, it suppressed EAE [4], this molecule was explored for its possible therapeutic effect, although its mechanism of action is still not fully known [2]. GA’s mechanism of action encompasses both anti-inflammatory properties (such as promoting a shift toward a Th2 pattern for reactive T cells, reducing production of inflammatory cytokines and enhancing T regulatory cells and anti-inflammatory cytokines [5,6,7,8]) as well as neuroprotective properties (such as increasing levels of neurotrophic factors, favoring axonal protection and possibly reducing glutamate-mediated neurotoxicity [9,10,11]). Among biological characteristics of GA, its immunogenicity needs to be highlighted for the purposes of this review: in a high proportion of patients treated with GA a low but significant titer of anti-GA antibodies (anti-GA) is produced [12]. Anti-GAs do not interfere with its efficacy [13].
After GA was tested in several studies over 25 years, two large randomized controlled trials have been performed testing GA in patients with MS. In the US glatiramer acetate study [14], a 29% reduction of the annualized relapse rate (ARR) favoring GA over placebo was observed, and in the European–Canadian MRI study [15], GA-treated patients showed a reduction of roughly 70% of gadolinium-enhancing (Gd+) lesions versus placebo. These studies ultimately led to GA approval as a DMD for MS in 2001.
Fifteen years later, GA’s patent expired, leading to great interest in the development of new members of its class. Not all glatiramoids have shown favorable outcomes in the treatment of MS [1]. In the present review, we will discuss the regulatory and statistical background of generic drugs for MS, and we will present preclinical and clinical evidence of Copemyl®, a new glatiramoid, 20 mg administered subcutaneously (sc) daily, and discuss its equivalence to former GA in terms of efficacy and safety profile. This article is therefore based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Regulation of Generic Drugs and Biosimilars and the Case of Non-Biological Complex Drugs
A generic medicinal product is defined as a product which has the same qualitative and quantitative composition in active substances and the same pharmaceutical form as the reference medicinal product, and whose bioequivalence with the reference medicinal product has been demonstrated by appropriate bioavailability studies [16].
A biosimilar drug is a drug similar to a biological drug, i.e., according to the European Medicines Agency (EMA) definition: “A product, the active substance of which is a biological substance. A biological substance is a substance that is produced by or extracted from a biological source and that needs for its characterization and the determination of its quality a combination of physicochemical–biological testing, together with the production process and its control” [16].
There are several drugs that are neither classic small molecules nor biological substances. These drugs are called non-biological complex drugs (NBCDs) and consist mainly of therapeutic proteins; for instance: liposomal drugs, iron carbohydrate drugs (“iron-sugars”), and drugs belonging to the category of glatiramoids [17, 18]. The regulation of biosimilars is not applicable to those products, as they are not the originator a biological substance. On the other hand, the classical paradigm for the marketing of a generic drug cannot be used for NBCDs either, mainly because of the complexity of their structure that impairs classical structure analysis and pharmacokinetic studies [19,20,21]; therefore, these products resist regulation of their own.
The EMA regulates the approval of NBCDs and their marketing under Article 10(3) of Directive 2001/83/EC [16]. Article 10(3) does not impose the new drug and the originator to be identical, but states that for the approval of the new version, results of appropriate preclinical testing or clinical trials shall be provided. Applications for NBCDs will thus rely in part on the results of preclinical tests and clinical trials for the reference product, and in part on new data for the new version of the drug. Specifically, for new versions of GA such new data consisted in a randomized three-arm equivalence trial, including a placebo arm, a reference GA arm, and an arm treated with the new GA. Such an approach is in line with what was proposed in consensus meetings on therapeutic equivalence [17] and was agreed with the EMA Committee for Medicinal Products for Human Use (CHMP) [22] as further discussed. Given the current lack of specific EMA rules for the management of NBCDs registration, it should be considered—purely on practical grounds—that the EMA requires mandatory centralized procedures for the marketing authorization of biosimilar drugs. GA is not considered a biologic and therefore the EU biologics and biosimilar legislation and regulatory pathway is not applicable; in fact, GA copies were not registered through a centralized procedure.
The US Food and Drug Administration (FDA), unlike EMA regulation, does not require data from randomized trials to demonstrate sameness for complex active ingredients, such as GA, but, in addition to standards applied to evaluate all generic drug products, it requires “appropriate information” [23]. That further information for GA consisted mainly of demonstration of physicochemical equivalence and equivalent biological and immunological effects in EAE and were considered sufficient to approve another version of GA in 2015 [24]. Other glatiramoids purported to be generic GA are marketed in various countries for the treatment of MS patients [15]. Table 1 summarizes all different glatiramoids developed so far.
Methodology of Equivalence Trials
Trials to test the clinical efficacy of generic drugs are necessarily equivalence trials, aimed at demonstrating that the drug is equivalent to the original compound. This is the first critical issue to be taken into account: a non-inferiority condition is not sufficient and the two drugs must have the same efficacy. Assessing equivalence of two drugs is generally more difficult than assessing superiority of one drug over another, and it requires larger numbers of patients. The reason for that lies in the definition of “equivalence”. In an equivalence trial the null hypothesis (that we want to reject) is that the new drug has an inferior (or a superior) efficacy than the original drug, while the alternative hypothesis is that the new drug has the same efficacy as the original one [25]. Therefore, the first step for designing an equivalence trial is to define what we mean for “equivalent” and this definition implies the need to set the so-called equivalence margin. The equivalence margin is the interval of differences (around zero) between the new and the original drug that we can consider clinically not relevant, so that if the difference detected in the trial lies within this interval we can declare that the two drugs are clinically equivalent. It is obvious that the equivalence margin must be narrow and in any case narrower than the interval used to declare superiority [26]. If, for example, in a study in MS we define a new drug superior to the standard drug if the former reduces the relapse rate of 30% more than the latter, the equivalence margin for two drugs in MS must be narrower than 30%. We could tolerate, for example, a difference in relapse rate of 15% as a difference with no clinical relevance, so that we could decide to set the equivalence margin as [−15%, +15%]. The narrower the interval, the higher the likelihood that the two drugs are equivalent, but the larger the sample size needed [25].
Preclinical Data
The synthesis of Copemyl is based on the same chemistry as the synthesis published for GA, which results in the complex heterogenous mixture of random polypeptide chains ranging from 20 to 200 amino acids with an average of 60 amino acids in length. Consequently, it has been estimated that any of more than 1029 different potential polypeptide sequences could be found in GA and in Copemyl [27]. The manufacturer of Copemyl has performed an extensive physicochemical and biological characterization program comparing the active substance present in Copemyl and GA, using a panel of chemical and biological assays, including primary structure evaluation, complex structure evaluation, UV spectroscopy, and capillary isoelectric focusing [27].
Bioequivalence between Copemyl and GA was tested via several bioassays. Copemyl and GA were compared in a study evaluating their effect in modulating gene expression in the human monocytic THP-1 cell line [28]. In this study five Copemyl batches were compared to five GA batches using the GeneChip® Human GenomeU133 Plus 2.0 Array (Thermo Fisher Scientific Inc.) for analysis of genome-wide expression, evaluated by microarray after 6 h exposure of THP-1 cells in vitro to either Copemyl or GA, showing that the variability of the modulated genes of the Copemyl batches was similar to that of the GA batches (specifically, a total of 52 probe sets were significantly up- or downregulated by Copemyl and 60 by GA, compared to vehicle-treated samples. The variability of the modulated genes of the Copemyl batches was similar to that of the GA batches). Another study focused on induction and activation of glatiramer-specific T cells in EAE [29]. Mice were immunized with different batches of either GA or Copemyl, and 10 days later lymphocytes were obtained from the draining lymph nodes. The isolated cells were cultured and restimulated with increasing doses of GA in vitro. GA-specific T cells respond to in vitro (re)stimulation with increased proliferation and cytokine—including interleukin-2 (IL-2)—production [30]. IL-2 levels were quantified and the GA-dependent IL-2 secretion served as a parameter for bioactivity. The bioactivities ranged between 61.6% and 94.3% for Copemyl batches and between 56.3% and 94.8% for GA batches, thus confirming analogous immunomodulatory properties. Comparative toxicity studies were performed in rats [27] to whom Copemyl and GA were administered subcutaneously by daily injection showing analogous local reactions and liver effects, with no difference in frequency and severity of these effects in rats treated with Copemyl and rats treated with GA.
Clinical Data
Copemyl was evaluated in patients with MS in two trials: the 9-month GATE trial [31] and its 15-month extension [32]. The GATE study is a randomized, double blind active and placebo-controlled equivalence trial conducted in 17 countries in 2011–2013 and published in 2015. Patients included were naïve to GA, had mild to moderate disability [Expanded Disability Status Scale (EDSS) [33] of 0–5.5], and had an active disease (a relapse in the previous year, and 1–15 Gd+ lesions at the screening scan). A total of 796 patients were randomized to either Copemyl 20, GA, or placebo, with a ratio of 4.3:4.3:1. The primary endpoint was the number of Gd+ lesions during months 7–9, with an equivalence margin (EM) tailored considering results of the former GA Euro-Canadian study [15], as further discussed. Equivalence required both statistically significant superiority to placebo and a 95% CI within predefined equivalence margins for the ratio of Gd+ lesions between Copemyl and GA. Most relevant baseline characteristics of the randomized patients (and comparison with the reference GA trials) are illustrated in Table 2, while Fig. 1 displays results of the primary endpoint. MRI of Copemyl- and GA-treated patients showed a mean number of Gd+ lesions lower than those administered placebo (ratio 0.488; 95% CI 0.365–0.651; P < 0.001), confirming study sensitivity. The estimated ratio of Copemyl to GA Gd+ lesions was 1.095 (95% CI 0.883–1.360), which fell within the predefined equivalence margin; therefore, the authors concluded equivalence between Copemyl and GA. ARR, though calculated over 9 months, did not differ in the three arms [0.31 (95% CI 0.20–0.48) for Copemyl, 0.40 (95% CI 0.26–0.62) for GA, and 0.38 (95% CI 0.22–0.66) for placebo]. In the three groups mean EDSS score was stable, percentage of relapse-free or disease-activity-free patients did not differ, and no statistically relevant difference in new T2 MRI lesions was shown. Adverse events (AEs) were evenly reported in all arms of the study; trademark GA adverse events such as injection site reactions (ISR) and immediate post-injection reactions occurred (PIR) in the same proportion of patients assigned to GA or Copemyl; namely ISR were reported by 16.4% of the patients in the Copemyl arm and 17.4% of those in the GA arm, while PIR occurred respectively in 6.8% of the patients for Copemyl and 5% of the patients for GA.

This figure is slightly modified in terms of color and with supplementary notes from [31]. Copyright permission from American Medical Association, owner of the rights of the article, was obtained on June 16, 2017
Primary endpoint of the GATE trial [31]: left main number of Gd+ lesions in months 7–9 in the three arms of the study and right predefined equivalence margins of n-GA versus GA.
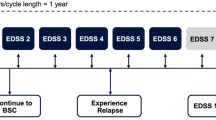
Patients enrolled in the GATE study were offered the possibility to continue in a 15-month open-label extension trial, whose results have been recently published [32]. Of the 796 patients of the GATE study, 728 agreed to participate in the extension and all were treated with Copemyl. Of these, 670 completed the trial (92%). For analysis purposes subjects enrolled into the extension trial were divided into three arms: patients treated with placebo in the GATE trial (pbo/Copemyl, n = 81), patients assigned to GA arm in the GATE trial (GA/Copemyl, n = 323), and patients randomized to Copemyl in the GATE trail (Copemyl/Copemyl, n = 324). MRI conducted at month 12, 18, and 24 from GATE trial randomization showed a similar mean number of Gd+ lesions for GA/Copemyl patients and Copemyl/Copemyl patients (0.6–0.7 in both groups), while pbo/Copemyl patients showed a higher mean number of Gd+ lesion at the 12-month scan (1.7), declining to 0.7 and 0.9 at months 18 and 24, respectively (Fig. 2).The change in the number of new T2 lesions was similar in the Copemyl/Copemyl and GA/Copemyl groups but higher in the placebo/Copemyl group. Changes in other MRI parameters (T2 lesion volume, T1 hypointense lesion volume, and brain volume) were similar in the Copemyl/Copemyl and GA/Copemyl groups and larger in the placebo/Copemyl group. In parallel to what was observed in the core trial, clinical outcomes, in particular ARR (0.21, 0.24, and 0.23 for Copemyl/Copemyl, GA/Copemyl, and placebo/Copemyl, respectively) did not differ among the three groups. The authors state that these figures are comparable to the 2-year ARR reported in recent clinical trials with GA. Injection-related AEs were similarly low in the GA/Copemyl group and Copemyl/Copemyl group (respectively 0.9% and 1.2% for injection site reactions and 0.9% and 2.2% for immediate post-injection reactions), while injection site reactions were reported in 9.9% and immediate post-injection reactions in 1.2% of patients switching from placebo to Copemyl. Anti-GA antibodies were tested throughout the whole duration of both core and extension study (Fig. 3). During the 9-month double blind study, the anti-GA-positive proportion of patients and antibody titer were similar in GA- and Copemyl-treated patients. Interestingly, when GA patients switched to Copemyl, their antibody titer remained equivalent to those of patients originally randomized to Copemyl. These findings allowed the authors to conclude that efficacy and safety of Copemyl are maintained over a certain time and that switching from GA to Copemyl might be done without loss of efficacy, safety, or tolerability.

This figure is slightly modified in terms of color and with supplementary notes from [32]. This work is licensed under the Creative Commons Attribution-NonCommercial 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/3.0/ or send a letter to Creative Commons, P.O. Box 1866, Mountain View, CA 94042, USA

This figure is slightly modified in terms of color and with supplementary notes from [32]. This work is licensed under the Creative Commons Attribution-NonCommercial 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/3.0/ or send a letter to Creative Commons, P.O. Box 1866, Mountain View, CA 94042, USA
Discussion
Both the GATE study and its extension provide evidence in support of the equivalence between Copemyl and GA. In the GATE study, the equivalent margin (EM), which clearly is of pivotal importance in such studies, was decided taking into consideration the results of the pivotal studies of GA vs placebo. As previously described, the EM is defined as the largest difference between the originator and the test drug that can be judged as clinically acceptable, and should be no larger than differences observed in the superiority trial of the originator [26]. Therefore, as in the Euro/Canadian trial in months 7–9 the mean number of Gd+ lesions in placebo-treated patients was 1.75 times higher than in GA-treated patients [15], for the GATE trial the upper limit of the EM was set at 50% of that value, i.e., 1.375; consequently, the lower limit was set at 0.727, symmetrically in the log scale. The value of 50% was chosen because it is possible to estimate the expected effect on relapses from an observed effect on MRI lesions, according to the Sormani equation [34, 35]. An effect on MRI lesions as relative risk compared to the originator (RR) of 0.727 (reduction of 27.5%) translates into an effect on relapses as RR 0.825 (reduction of 17.5%); such a difference was considered non-clinically relevant. Furthermore, in equivalence trials it is crucial that an assay sensitivity is provided, as it quantifies to the ability of the trial to detect a difference between treatments, if such a difference exists [36].
Authors of the GATE trial state that sample size calculation (with a dropout rate of 12% allowed) was performed to ensure a 98% power to demonstrate study sensitivity, a 92% power to show equivalence of GA and Copemyl, and a 90% power to show both sensitivity and equivalence. Results from the GATE trial were bolstered by the extension trial, in which MRI results are fully consistent with the hypothesis of the equivalence between Copemyl and GA. Furthermore, anti-GA antibodies monitoring throughout the whole duration of the open label and the extension trial provided interesting information. The incidence and titer of anti-GA were comparable with both forms of GA. Moreover, switching from GA to Copemyl did not affect anti-GA titers. These results demonstrate that GA and Copemyl have comparable immunogenicity, which is of great relevance when it comes to evaluating interchangeability of complex or biologic drugs [37, 38]. To our knowledge, this is the only new version of GA whose immunogenicity has been evaluated in MS patients.
Even with adequate EM and study sensitivity, and with immunological evidence in support, a few drawbacks of the GATE study need to be addressed: (1) the fact that the primary endpoint was not a clinical endpoint but an MRI surrogate of clinical activity, (2) the fact that over the observation period no differences in terms of ARR appeared in the three arms of the study.
The choice of the primary endpoint was de facto suggested by EMA, after the manufacturers of Copemyl on several occasions sought EMA scientific advice as regards the type of new data required [22]. In fact, the EMA CHMP stated that MRI measures are acceptable to detect effect and establish equivalence of two products containing GA in a shorter study duration, in cases that the quality data indicate a high level of similarity [22]. Also, the current CHMP guideline on multiple sclerosis [39] declares MRI endpoints to be sufficient for demonstrating similarity of two products in the context of biosimilar and generic applications. Anyway, such endpoints could be perceived as puzzling, as to date MRI activity has not been the primary endpoint in any phase III trial in MS. However, over the last years there has been mounting evidence of a strong association between MRI activity and clinical activity. Two meta-analyses of studies encompassing several DMDs demonstrated that in MS relapse rate strongly correlates with Gd+ lesions, and, more importantly, that the magnitude of the effect of the drug on MRI parameters can predict the magnitude of the benefit in terms of ARR [34, 35]. As far as it concerns specifically GA, the correlations between MRI and clinical activity parameters are particularly robust, stronger than what was observed, for instance, in interferon-beta (IFN-β) studies. In fact, trials comparing GA and IFN-β head to head showed a similar efficacy on relapses, whereas the effect on MRI activity was somewhat less pronounced for GA [40,41,42] and meta-analysis confirmed these results [43]. These observations were summarized in a paper published in Nature Reviews Neurology stating that for equivalence trials on a drug such as glatiramer acetate, with almost the same magnitude of effect on MRI activity and relapse rate, an MRI-based approach seems justified [44].
In the GATE trial, the apparent lack of effect of both GA and Copemyl on relapse rate may result from multiple factors. The authors suggest that the trial was not designed or powered to show relapse rate reduction: the placebo group consisted only of 84 subjects and therefore the expected power to demonstrate an ARR reduction was less than 30%. In addition to that, the ARR of the placebo group was low, both at baseline (if compared to the US and the Euro-Canadian trial) and during the trial. This observation is not at all unexpected, as it is well known that ARR in the placebo groups of phase III trials in MS have consistently decreased over the years [45]. It suggests that subjects included in the recent trials have less aggressive forms of MS than patients included in the early ones; thus, in order to detect an effect on ARR, larger sample trials are required in order to compensate for overall low event rates. Furthermore, the duration of the GATE trial was only 9 months. In fact, evidence from a trial with GA in 481 patients with a first demyelinating event (CIS) at high risk of developing MS [46] suggests that the effect of GA on clinical activity emerges over placebo after at least 6 months of treatment, suggesting that a 9-month observation time might have played a role in not allowing an effect on relapses to emerge. Anyway, even taking these considerations into account, the lack of effect on relapses of both active compounds is somehow puzzling.
The results of the extension study confirmed the effect of Copemyl on MRI activity as patients previously treated with placebo showed a reduction of T1 Gd-enhancing lesions and T2 lesions at the end of the trial. The effect on MRI parameters was similar in the two cohorts of patients previously treated with Copemyl or GA: in other words, the effect on MRI was maintained in patients who switched from GA to Copemyl.
The rate of AEs in both GATE and its extension trials, namely injection site reaction and post-injection systemic reaction, was lower than that reported in the trial of GA in CIS mentioned above [46] and, although it could probably have been underestimated, it was even in patients treated with Copemyl or GA.
Conclusions
Many medications are currently available for the treatment of MS, with the clear advantage of offering many options and the objective to tailor the therapy to patient characteristics and to provide higher efficacy for breakthrough disease. As a result, though, the costs of MS treatment have increased, as the prices of highly effective MS DMDs are generally higher than those of platform therapies. Efforts should be made to address this question, which potentially limits a wider access to some therapies. One probable reason for the skyrocketing costs of MS DMDs is that DMDs present on the market for decades have never faced price competition from lower-cost equivalent drugs [47]. However, the effect of the availability of a generic DMD on the costs of MS therapy is still unclear, because it will heavily depend upon its price and the extent of its use, i.e., the extent of how neurologists and patients are confident that the new drug has comparable efficacy, safety, and tolerability as the brand drug. Clinicians will therefore ask to be reassured on the comparable safety and efficacy profile of new formulations. A debate is ongoing on these issues, in particular on two additional aspects: how definitions of regulatory guidelines are exhaustive, clear, indisputable [48], and how new formulations are actually identical to the original medication, an aspect particularly important for NBCDs, and especially for glatiramoids [18, 49]. Glatiramoids are in fact immunomodulators, altering pathogenetic immune mechanisms, but also implying possible risks such as immunotoxicity, induction of autoimmune disorders, and lack of efficacy [50]. As glatiramoids are not amenable to conventional methods of demonstrating bioequivalence, according to the same author [50], the only way to test their safety and efficacy is to produce robust data from clinical trials. The evidence presented in this review should reinforce the confidence of clinicians as far as it concerns Copemyl, as published data in fact suggest equivalence of Copemyl and GA on the basis of immunological and MRI outcomes, thus making Copemyl a viable option, along with interferons or teriflunomide among others, for patients suffering with mild to moderate forms of MS. Anyway, the drawbacks pertaining to clinical data should be taken into account, and there is a need for post-marketing evidence comparing the two compounds in everyday practice and further confirming their equivalence.
References
Johnson KP. Glatiramer acetate and the glatiramoid class of immunomodulator drugs in multiple sclerosis: an update. Expert Opin Drug Metab Toxicol. 2010;6(5):643–60.
Comi G, Amato MP, Bertolotto A, et al. The heritage of glatiramer acetate and its use in multiple sclerosis. Mult Scler Demyelin Disord. 2016;1:6.
Arnon R. The development of Cop 1 (Copaxone), an innovative drug for the treatment of multiple sclerosis: personal reflections. Immunol Lett. 1996;50(1–2):1–15.
Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971;1(4):242–8.
Aharoni R, Teitelbaum D, Sela M, Arnon R. Bystander suppression of experimental autoimmune encephalomyelitis by T cell lines and clones of the Th2 type induced by copolymer 1. J Neuroimmunol. 1998;91(1–2):135–46.
Oreja-Guevara C, Ramos-Cejudo J, Aroeira LS, Chamorro B, Diez-Tejedor E. TH1/TH2 cytokine profile in relapsing-remitting multiple sclerosis patients treated with glatiramer acetate or natalizumab. BMC Neurol. 2012;12:95.
Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naïve CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J Neuroimmunol. 2009;216(1–2):113–7.
Aharoni R, Eilam R, Stock A, et al. Glatiramer acetate reduces Th-17 inflammation and induces regulatory T-cells in the CNS of mice with relapsing-remitting or chronic EAE. J Neuroimmunol. 2010;225(1–2):100–11.
Arnon R, Aharoni R. Neurogenesis and neuroprotection in the CNS–fundamental elements in the effect of glatiramer acetate on treatment of autoimmune neurological disorders. Mol Neurobiol. 2007;36(3):245–53.
Skihar V, Silva C, Chojnacki A, et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc Natl Acad Sci USA. 2009;106(42):17992–7.
Gentile A, Rossi S, Studer V, et al. Glatiramer acetate protects against inflammatory synaptopathy in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2013;8(3):651–63.
Farina C, Vargas V, Heydari N, Kumpfel T, Meinl E, Hohlfeld R. Treatment with glatiramer acetate induces specific IgG4 antibodies in multiple sclerosis patients. J Neuroimmunol. 2002;123(1–2):188–92.
Johnson KP, Teitelbaum D, Arnon R, The US Phase III Copolymer 1 Study Group, et al. Antibodies to copolymer do not interfere with its clinical effect. Ann Neurol. 1995;38:973.
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45(7):1268–76.
Comi G, Filippi M, Wolinsky JS, European/Canadian Glatiramer Acetate Study Group. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol. 2001;49(3):290–7.
EU Definition (Directive 2001/83/EC, Annex I (=Directive 2003/63/EC)) of a biological medicinal product.
Schellekens H, Klinger E, Mühlebach S, Brin JF, Storm G, Crommelin DJ. The therapeutic equivalence of complex drugs. Regul Toxicol Pharmacol. 2011;59(1):176–83.
Crommelin DJ, Shah VP, Klebovich I, et al. The similarity question for biologicals and non-biological complex drugs. Eur J Pharm Sci. 2015;76:10–7.
Crommelin DJ, Bermejo T, Bissig M, et al. Pharmaceutical evaluation of biosimilars: important differences from generic low-molecular weight pharmaceuticals. Eur J Hosp Pharm Sci. 2005;1:11–7.
Schellekens H. Follow-on biologics: challenges of the ‘next generation’. Nephrol Dial Transplant. 2005;20:31–6.
Baumann A. Nonclinical development of biopharmaceuticals. Drug Discov Today. 2009;14:1112–22.
EMA: decentralised procedure RMS final assessment report. CMDh/200/2007 Rev. 5 December 2013.
US Food and Drug Administration (2015) FDA approves first generic copaxone to treat multiple sclerosis. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm443143.htm. Accessed 15 Apr 2015.
Citizen petition denial letter from CDER to Teva Pharmaceuticals. http://www.regulations.gov/#!documentDetail;D=FDA-2015-P-1050-0012. Accessed 16 Apr 2015.
Wellek S, Blettner M. Establishing equivalence or non-inferiority in clinical trials: part 20 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(41):674–9.
Njue C. Statistical considerations for confirmatory clinical trials for similar biotherapeutic products. Biologicals. 2011;39(5):266–9.
College ter Beoordeling van Geneesmiddelen (CBG) public assessment report (PAR) Scientific discussion Glatirameeracetaat Mylan 20 mg/ml, solution for injection, pre-filled syringe (glatiramer acetate) NL/H/3213/001/DC. 6 June 2016.
Arends R. Gene expression analysis between 5 GTR (20 mg/mL) batches and 5 Copaxone® (20 mg/mL) batches in THP-1 cells. Dossier NDR.NL03.39931 (1.0).
Weijts F (2010) Research analytical study report on bioactivity of glatiramer acetate (GTR) towards induction and activation of glatiramer-specific T cells. Dossier RASR.NL03.GTR.10.010.01.
Aharoni R, Teitelbaum D, Arnon R. T suppressor hybridomas and interleukin-2-dependent lines induced by copolymer 1 or by spinal cord homogenate down-regulate experimental allergic encephalomyelitis. Eur J Immunol. 1993;23:17–25.
Cohen J, Belova A, Selmaj K, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72(12):1433–41.
Selmaj K, Barkhof F, Belova AN et al (2017) Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. doi:10.1177/1352458516688956.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33(11):1444–52.
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65(3):268–75.
Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol. 2013;12(7):669–76.
Choice of control group and related issues in clinical trials. ICH Topic E 2000; 10.
Tóthfalusi L, Endrényi L, Chow SC. Statistical and regulatory considerations in assessments of interchangeability of biological drug products. Eur J Health Econ. 2014;15:S5–11.
Wu LC, Chen F, Lee SL, Raw A, Yu LX. Building parity between brand and generic peptide products: regulatory and scientific considerations for quality of synthetic peptides. Int J Pharm. 2017;518(1–2):320–34.
EMA/CHMP/771815/2011, Rev. 2.
Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs glatiramer acetate in relapsing MS disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–14.
O’Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889–97.
Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73(3):327–40.
La Mantia L, Di Pietrantonj C, Rovaris M, et al. Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2016;24:11.
Sørensen PS. Multiple sclerosis. Generic glatiramer acetate—a step toward cheaper MS drugs? Nat Rev Neurol. 2016;12(1):5–6.
Stellmann JP, Neuhaus A, Herich L et al. Placebo cohorts in phase-3 MS treatment trials—predictors for on-trial disease activity 1990–2010 based on a meta-analysis and individual case data. PLoS One. 2012;7(11):e50347.
Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9700):1503–11.
Bourdette D, Hartung D. Equivalence of glatiramer acetate generics with branded glatiramer acetate in efficacy and cost for the treatment of multiple sclerosis. JAMA Neurol. 2015;72(12):1411–3.
Garattini L, Padula A. Why EMA should provide clearer guidance on the authorization of NBCDs in generic and hybrid applications. Expert Rev Clin Pharmacol. 2017;10(3):243–5.
Crommelin DJ, de Vlieger JS, Weinstein V, et al. Different pharmaceutical products need similar terminology. AAPS J. 2014;16(1):11–4.
Nicholas JM. Complex drugs and biologics: scientific and regulatory challenges for follow-on products. Drug Inf J. 2012;46(2):197–206.
Acknowledgements
Sponsorship for article processing charges was funded by Mylan BGP Products Srl. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, had full access to all data and versions of the manuscript, and take complete responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Disclosures
Pietro Annovazzi received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Merck Serono, Biogen, Teva, Sanofi-Aventis, Almirall, Roche, and Novartis. Antonio Bertolotto received honoraria for serving on the scientific advisory boards of Biogen, Merck, Mylan, Sanofi-Genzyme, and received speaker honoraria from Biogen, Genzyme, Novartis, TEVA; his institution has received grant support from Almirall, Bayer, Biogen, Genzyme, Merck, Novartis, TEVA, from the Italian Multiple Sclerosis Society, Fondazione Associazione Ricerca Biomedica ONLUS, and San Luigi ONLUS; his institution has received research support from Admirall, Biogen, Bayer, Merck, Sanofi-Genzyme, Novartis, Teva, from the Italian Multiple Sclerosis Society, Fondazione Associazione Ricerca Biomedica, and San Luigi Onlus. Vincenzo Brescia Morra received funding for travel, speaker honoraria, and research support from Sanofi-Aventis, Bayer Schering Pharma, Merck Serono, Biogen Idec, Sanofi-Genzyme, Novartis. Claudio Gasperini received research support from TEVA and speakers bureau from Merck, Novartis, Genzyme, Biogen, Teva. Enrico Montanari received grants from Merck, Biogen, Teva, Novartis, Genzyme, and Almirall. Pierluigi Navarra was funded by Mylan Italia for payment for expertise on a regulatory matter. Francesco Patti served on scientific advisory boards for Almirall, Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, and TEVA; he served on speakers’ bureau for Almirall, Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, and TEVA; he received research support from MIUR, ISS, and FISM. Maria Pia Sormani received consulting fees from TEVA, Genzyme, Roche, Novartis, Biogen, Merck serono, GeNeuro, Medday. Angelo Ghezzi received grants from Merck, Biogen, Teva, Novartis, Genzyme, and Almirall.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D3E8F06073CC394F.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Annovazzi, P., Bertolotto, A., Brescia Morra, V. et al. A Comprehensive Review on Copemyl® . Neurol Ther 6, 161–173 (2017). https://doi.org/10.1007/s40120-017-0079-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-017-0079-3