Abstract
Introduction
A high proportion of Canadian patients with acute myocardial infarction (AMI) do not achieve the threshold low-density lipoprotein cholesterol (LDL-C) levels recommended by the Canadian Cardiovascular Society in 2021. This increases the risk of subsequent atherosclerotic cardiovascular disease (ASCVD) events. Here, we assess LDL-C levels and threshold achievement among patients by lipid-lowering therapies (LLT) received post-AMI.
Methods
A retrospective cohort study of patients identified with AMI between 2015 and 2019 was conducted using administrative health databases in Alberta, Canada. Patients were grouped by their highest-intensity LLT post-AMI (proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) + another LLT; PCSK9i alone; ezetimibe + statin; statins (high, moderate, low intensity); or ezetimibe alone), and available LDL-C levels were examined in the year before and after LLT dispense date.
Results
The cohort included 15,283 patients. In patients on PCSK9i + LLT, the median [95% confidence interval (CI)] LDL-C levels decreased from 2.7 (2.3–3.4) before to 0.9 (0.5–1.2) mmol/l after treatment, the largest decrease among treatment groups. In the ezetimibe + statin and high-intensity statin groups, median (95% CI) values after treatment were 1.5 (1.5–1.6) and 1.4 (1.4–1.4) mmol/l, respectively. The proportion of patients below the 1.8 mmol/l threshold increased by 77.7% in the PSCK9i + LLT group after treatment, compared to 45.4 and 32.4% in the ezetimibe + statin and high-intensity statin groups, respectively.
Conclusions
Intensification with PCSK9i in AMI patients results in a greater proportion of patients achieving below the recommended LDL-C threshold versus statins and or ezetimibe alone. Increased focus on achieving below the LDL-C thresholds with additional LLT as required may benefit patient cardiovascular outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
A high proportion of Canadian patients with myocardial infarction (MI) fail to achieve the threshold low-density lipoprotein cholesterol (LDL-C) levels recommended by the Canadian Cardiovascular Society guidelines in 2021, which can result in increased risk of subsequent atherosclerotic cardiovascular disease (ASCVD) events. |
Intensification of lipid-lowering therapies (LLT) with proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) have shown a large benefit in lowering LDL-C levels in patients with recent MI. |
A real-world study on LDL-C levels and guideline threshold achievement in relation to LLT has not been conducted exclusively in post-acute myocardial infarction (AMI) patients, who are known to have greater risk of subsequent ASCVD events. |
What was learned from this study? |
Results from this study found that the time to treatment was much longer for patients receiving PCSK9i and there was a higher number of ASCVD events between their AMI and the start of their PCSK9i treatment. |
PCSK9i treatment in combination with other LLT provides the greatest reductions in LDL-C and guideline threshold achievement, thereby reinforcing the effectiveness of intensifying LLT in patients at high cardiovascular risk, as put forth by international guidelines. |
Introduction
Acute myocardial infarction (AMI) has a high burden in Canada, evidenced by higher rates for subsequent major adverse cardiovascular events in AMI patients relative to atherosclerotic cardiovascular disease (ASCVD) patients in general[1]. Despite this, a high proportion of patients with myocardial infarction (MI) may not receive lipid-lowering therapy (LLT) intensification post-MI and may not reach threshold low-density lipoprotein cholesterol (LDL-C) levels, [2] leaving patients at risk for subsequent ASCVD events and cardiovascular (CV) mortality [3, 4].
LDL-C reduction is generally recommended as a target for primary and secondary ASCVD prevention. The American Heart Association (AHA) recommends patients with very high risk ASCVD achieve below the LDL-C threshold of 1.8 mmol/l [5]. A recent consensus statement from the American College of Cardiology has recognized the clinical benefit of non-statin therapies in very high risk ASCVD patients to substantially lower LDL-C and CV outcomes in patients on statins, and therefore recommends a threshold below 1.4 mmol/l for considering therapeutic intensification [6]. The European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) recommend the more aggressive LDL-C goal of < 1.4 mmol/l for very high risk ASCVD patients, and < 1.0 mmol/l for patients with very high CV risk and a recurrent acute coronary syndrome (ACS) event within the preceding 2 years [7]. The strong emphasis on reducing LDL-C levels is to decrease the risk of future CV events, such as AMI and CV death. In 2021, the Canadian Cardiovascular Society Dyslipidemia guidelines were updated to lower the recommended LDL-C threshold for ASCVD patients to 1.8 mmol/l [3]. These guidelines state that in patients treated with maximally tolerated statins who remain above this threshold, intensification with ezetimibe and/or proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) is required. Further, in those with LDL-C above 2.2 mmol/l, it might be preferable to consider a PCSK9i as second-line therapy because treatment with ezetimibe alone would not be sufficient to achieve thresholds [3].
Patients with recent MI are shown to derive a large benefit from intensification of statin therapy with the additional use of a PCSK9i [3]. The PCSK9i monoclonal antibodies evolocumab and alirocumab were approved in Canada in 2015, and are currently indicated as adjuncts to diet and standard-of-care therapy to reduce the risk of CV events in adult patients with ASCVD by further lowering LDL-C levels. Recent real-world studies have demonstrated that PCSK9i can safely and effectively lower LDL-C levels in Canadians with atherosclerotic disease, consistent with larger clinical trials [8,9,10]. To our knowledge, a real-world study exclusively in post-AMI patients known to have greater risk of subsequent ASCVD events has not been conducted.
The purpose of this study was to assess LDL-C levels and guideline threshold achievement among patients with AMI receiving LLT, based on the highest intensity LLT prescription dispense(s) received post-AMI. Additionally, we explored the change in LDL-C before and after LLT among a subset of patients with before and after LDL-C measures available.
Methods
Study Design and Data Sources
A retrospective cohort study was conducted in Alberta, Canada, using population-level, province-wide administrative health data (see Supplementary Material Table 1 for data sources). The study complies with the Declaration of Helsinki and was approved by the Health Research Ethics Board of Alberta—Community Health Committee (HREBA-CHC).
Study Cohort
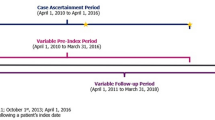
The cohort consisted of patients (≥ 18 years of age) with AMI during the case ascertainment period (April 1, 2015 to March 31, 2019). AMI was defined as the first inpatient hospitalization with the codes I21 or I22 from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada [11]. Further for cohort inclusion, patients must have had at least one LLT prescription post-AMI between April 1, 2015 and March 31, 2020 and one LDL-C test (with triglycerides ≤ 4.5 mmol/l) within 365 days before or after patients started their highest intensity LLT post-AMI. Included patients resided in Alberta, Canada, on their AMI date and the start of their highest intensity LLT. Based on the Friedewald formula [12], patients were excluded if they only had LDL-C tests with triglycerides > 4.5 mmol/l, which is inaccurate in patients with high levels of triglycerides. Patients with a PCSK9i prescription dispense before their AMI date were also excluded. The end of follow-up was the earliest of: [1] death, [2] leaving the province after the AMI date (deregistered from the Population Registry), or [3] the end of the study period (March 31, 2020).
Study Treatment Categorization
The LLT prescription dispenses post-AMI that were extracted included statins, ezetimibe, and PCSK9i (see Supplementary Material Table 2 for included Anatomical Therapeutic Chemical (ATC) codes). Statin intensities were categorized as low, moderate, or high based on AHA guidelines.[5] Treatment intensification was assessed using the Canadian Dyslipidemia Guidelines algorithm,[3] where ezetimibe ± PCSK9i are recommended add-ons to statin treatment where intensification is required. Treatment groups were categorized using the following hierarchy of mutually exclusive LLT intensification, ranked from highest to lowest based on intensity and degree of LDL-C lowering: (1) PCSK9i in combination with either statins, ezetimibe, or both; (2) PCSK9i alone; (3) ezetimibe + statin; (4) statin alone (high-, moderate-, or low-intensity); (5) ezetimibe alone. Combination regimens required a 90-day overlap for inclusion into those groups. Analyses were centered around the highest intensity LLT dispense date (referred to as the 'LLT dispense date’ hereon). For single regimens, this was when a patient started their treatment supply. For combination regimens, this was when a patient had all therapies of the regimen available.
Study Variables
Patient demographic characteristics captured at the AMI date and LLT dispense date included: age, sex, and Alberta Health Services geographic zone, categorized as urban (cities of Calgary and Edmonton) and rural (South, Center, and North zones).
Clinical characteristics of interest included: modified Charlson Comorbidity Index (CCI) score (using hospitalizations within 1 year, including diabetes as a comorbidity), [13,14,15,16] previous ASCVD event (see Supplementary Material Table 3 for included diagnostic codes), [11] number of previous ASCVD events (≥ 30-day gap between same events) in the 1 year pre-AMI and LLT dispense date (excluding AMI index event), severe hypercholesterolemia (LDL-C test result ≥ 5.0 mmol/l), closest laboratory values within 1 year of pre-AMI date and LLT dispense date (LDL-C, non-high-density lipoprotein cholesterol [non-HDL-C], triglycerides), and number of LDL-C tests post-AMI date over the follow-up period. Further, at the LLT dispense date, type of prescribing practitioner, and time (days) from AMI to LLT prescription dispense were extracted.
Post-AMI LDL-C test results in 3-month intervals were extracted within 12 months before and after the LLT dispense date (specified in Supplementary Figure 1). If there was more than one test within a 3-month interval, the highest LDL-C test result was used. The overall number of post-AMI LDL-C tests pre- and post-LLT dispense date were described, in addition to the sample size at each LDL-C timepoint. An additional analysis was conducted among a subset of patients with both a before and after LLT dispense date LDL-C test available.
Statistical Methods
All data analyses were descriptive in nature. Patient characteristics and LDL-C levels were analyzed using descriptive statistics for continuous and categorical variables [e.g., mean (standard deviation (SD)), median (interquartile range (Q1–Q3) or 95% confidence interval (CI)), etc.]. LDL-C levels before and after the LLT dispense date were described as the median of the entire year. The proportion of patients achieving LDL-C guideline thresholds among patients with available LDL-C tests in the 1 year before and after the LLT dispense date were examined using < 1.8 mmol/l, [3, 5] < 1.4 mmol/l, and < 1.0 mmol/l thresholds [7]. Among patients with a before and after LLT dispense LDL-C test available, the median (95% CI) percent change for patients in each LLT regimen was derived using the average of 3-month maxima LDL-C tests available in the six months before and after the LLT dispense date.
Results
Study Cohort and Patient Characteristics
Overall, 15,283 patients were included in the cohort (Supplementary Figure 2 shows the study flow chart). There were 6674 (24.7%) patients excluded for not having an eligible LDL-C test within 365 days of their LLT dispense date. At AMI date (Table 1), the mean (SD) age was 65.1 (12.1) years, patients were predominantly male (72.6%), and most lived in urban areas (62.9%). In the one-year prior to AMI events, 51.2% of patients had an ASCVD event and the median (Q1–Q3) number of prior ASCVD events was 1.0 (0.0–2.0). The closest LDL-C test result obtained pre-AMI showed the mean (SD) LDL-C was 2.6 (1.1) mmol/l and most patients (71.9%) had an LDL-C test result above the ≥ 1.8 mmol/l threshold. Of note, 836 (5.5%) patients had no LDL-C test identified in the year preceding their AMI date.
At LLT dispense date, most patients in each treatment group were male (> 56.6% per treatment group) and patients on PCSK9i in combination had the lowest mean (SD) age of 53.7 (12.8) years, while the highest of 74.5 (10.0) years was observed in the low-intensity statin group (see Supplementary Material Table 4 for patient characteristics at LLT dispense date). Overall, 96 patients were classified into the PCSK9i treatment groups, of which approximately 75% of patients were prescribed evolocumab (data not shown).
Median (Q1-Q3) LDL-C levels pre-LLT dispense date were 3.0 (2.2–4.2) mmol/l in PCSK9i alone patients and 3.1 (2.3–3.6) mmol/l in PCKS9i combination patients, which was greater than high-intensity statin patients [2.5 (1.8–3.3) mmol/l] and other statin groups (Supplementary Table 4). Most patients on PCSK9i received their prescription dispense from a specialist practitioner. The median (Q1–Q3) time in days between AMI date and dispense date was 387.5 (179.0–710.0) for PSCK9i alone and 324.0 (127.0–454.0) for PCSK9i in combination, in contrast to 121.0 (26.0–458.0) for ezetimibe + statin and 0.0 (0.0–7.0) for high-intensity statin groups (Fig. 1). In addition, the median (Q1–Q3) ASCVD events occurring in the year preceding LLT dispense date was 4.0 (2.0–6.0) for the PSCK9i combination group and 3.5 (2.0–6.0) for the PSCK9i alone group, which were markedly higher than the next hierarchical treatment group (eze + statin) of 2.0 (1.0–4.0). Post-AMI, 86.2% (n = 25/29) of PCSK9i combination patients and 78.5% (n = 51/65) of PCSK9i alone patients had LDL-C tests (Fig. 2), which was higher than other treatment groups, particularly the high-intensity statin group (15.2%; n = 1899/12,523). Generally, the number of LDL-C tests tended to be lower than the number of patients in each group in the year preceding the LLT dispense date (see Supplementary Material Table 5 for pre-LLT LDL-C levels and Supplementary Material Table 6 for post-LLT LDL-C levels across treatment groups).
Time to LLT dispense date from AMI date and the number of ASCVD events. AMI acute myocardial infarction, ASCVD atherosclerotic cardiovascular disease, Eze ezetimibe, LLT lipid-lowering therapy, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor, Q quartile. a The number of ASCVD events excludes the AMI index event, but could include pre-AMI events for those who received their highest-LLT within 1 year of AMI. ASCVD events of the same condition occurring within 30 days of each other were captured as the same event; but events of different conditions were considered separate events, regardless of the time between events. b Includes statin, ezetimibe, and ezetimibe plus statins
Median (95% CI) LDL-Ca in the year before and after LLT across treatment categories. CI confidence interval, Eze ezetimibe, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor. a The LDL-C values are an overall average of the combined highest test result available for each 3-month window pre- and post-LLT dispense date per patient. b Any LLT combination includes statin, ezetimibe, and ezetimibe plus statins
LDL-C Levels and Guideline Threshold Achievement
The net reduction in LDL-C in the year following LLT treatment was larger for PCSK9i regimens, specifically the PCSK9i combination group, than in other LLT categories (Fig. 2). The PCSK9i combination regimen demonstrated the greatest post-treatment LDL-C reduction to a median (95% CI) of 0.9 (0.5–1.2) from 2.7 (2.3–3.4) mmol/l. PCSK9i alone reduced LDL-C levels from 3.0 (2.5–3.2) to 1.4 (1.0–1.7) mmol/l. In contrast, ezetimibe + statin and high-intensity statin resulted in LDL-C levels of 1.5 (1.5–1.6) from 2.3 (2.2–2.3) and 1.4 (1.4–1.4) from 1.9 (1.9–2.0) mmol/l, respectively. The PCSK9i combination regimen also had the greatest improvement in the proportion of patients achieving all three guideline-recommended LDL-C thresholds, in the year following LLT dispense date with 85.7, 75.0, and 57.1% of patients achieving < 1.8, < 1.4, and < 1.0 mmol/l thresholds, respectively (Fig. 3). In contrast, the high-intensity statins alone resulted in 77.4, 52.2, and 21.1% of patients achieving < 1.8, < 1.4, and < 1.0 mmol/l thresholds, respectively.
Proportion (%) of patients with LDL-C below three thresholds ((a) 1.8 mmol//l (b) 1.4 mmol/l and (c) 1.0 mmol/l) before and after LLT treatment. LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor. Note: the values above the bar charts indicate the difference in the proportion of patients below the threshold before versus after the LLT dispense date (difference = after—before)
With respect to other lipoprotein lab tests of interest, the median non-HDL-C levels post-LLT dispense date were also lowest in the PCSK9i treatment groups. While triglycerides had minimal change post-LLT dispense date across most treatment groups, the largest decrease was observed in the PCSK9i combination group (see Supplementary Material Table 7 for lab tests pre- and post-LLT dispense date).
The analysis of patients with before and after LDL-C tests available showed similar trends to the primary analysis, with the largest median (95% CI) percent decrease in LDL-C levels of 66.5% (55.8–91.1) for the PCSK9i combination regimen and 52.5% (29.7–69.3) for the PCSK9i alone group (Fig. 4). In contrast, the median (95% CI) percent decrease was 28.4% (25.4–31.0) in the ezetimibe + statin group and 27.8% (24.2–31.0) in the high-intensity statin group.
Median (95% CI) percent change in LDL-C in the 6 months before and after LLT by treatment category among patients with a before and after LDL-C test available. CI confidence interval, Eze ezetimibe, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor. a The LDL-C values used are an overall average of the combined highest values of tests available obtained for each 3-month window pre- and post-LLT dispense date per patient. b Includes statin, ezetimibe, and ezetimibe plus statins
Discussion
The greatest reduction in median LDL-C levels was seen in PCSK9i-treated AMI patients compared to those treated with less intense therapeutic regimens. However, the time to treatment was much longer and these patients had a higher number of ASCVD events between their AMI and the start of their PCSK9i treatment.
A longer time to PCSK9i dispense date is expected given the typical course of therapeutic action for post-MI patients whereby high-(or moderate-) intensity statins are given in hospital prior to discharge and, at subsequent follow-ups, LDL-C is measured and treatments are intensified to ezetimibe and/or PCSK9i as required [3, 17]. However, due to the increased risk of recurrent events in the first year post-AMI, guidelines strongly recommend intensification as early as possible [7]. Indeed, in patients with ACS whose LDL-C levels are not at threshold and have been treated with maximally tolerated statins and ezetimibe, ESC/EAS guidelines recommend intensification to PCSK9i in hospital if possible [7]. Some payer requirements (e.g., ezetimibe trial before prescription; LDL-C tests) may also contribute to the time delay between the AMI event and PCSK9i initiation [18,19,20]. The high number of ASCVD events observed before starting PCKS9i could reflect the study design, whereby pre-AMI events may be included if the LLT was initiated within 1 year of the AMI and events following LLT dispense were not captured. However, given the median (Q1–Q3) time to initiate a PCSK9i in this study was 324.0 (127.0–454.0) days in combination and 387.5 (179.0–710.0) alone, it is also possible that these high-risk patients suffered additional ASCVD events while remaining above the LDL-C thresholds and awaiting appropriate LLT intensification. Our finding is consistent with a study in the United States that demonstrated an increasing cumulative incidence of CV events between AMI and PCSK9i initiation, where 8.0, 10.5, and 12.5% of patients had an ASCVD event at 90, 180, and 365 days post-AMI hospitalization discharge, respectively, before initiating a PCSK9i [21]. Together, these results emphasize the importance of earlier intervention with aggressive LDL-C management to prevent further adverse cardiac events, as recommended by the current international guidelines [3, 5,6,7].
In our study, patients treated with PCSK9i post-AMI demonstrated the greatest decrease in LDL-C levels, and, consistent with other studies, the ability to reach lower LDL-C levels was greater when PCSK9i was used in combination [20]. Patients treated with PCSK9i in combination had the highest proportion of patients achieving all guideline-recommended thresholds, versus patients treated with statin and/or ezetimibe. In a sub-analysis of recent MI patients from the ‘further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER)’ study, 92% of patients treated with evolocumab in combination with moderate to high intensity statins were able to achieve LDL-C levels below the 1.8 mmol/l threshold versus 18% of patients treated with moderate-to-high intensity statins alone [22]. Our results confirm that the majority of patients with intensification to PCSK9is in the real world, outside of a well-controlled clinical trial, may also be able to achieve below LDL-C thresholds. Notably, more than half of PCKS9i combination and one-third of PSCK9i alone patients achieved the < 1.0 mmol/l threshold, which is more than 50% higher than seen in other treatment groups. Although this analysis focused on LDL-C and does not report CV outcomes, the causal relationship between reductions in LDL-C and CV event rates is well established. Data from the Cholesterol Treatment Trialists’ Collaboration meta-analysis, assessing participant-level data from 26 randomized control trials, demonstrated that for every 1.0 mmol/l reduction in LDL-C, there is a corresponding 21% reduction in major coronary events and 10% reduction in mortality [23]. A lower achieved LDL-C has been significantly associated with a lower CV event rate, [23, 24] and the LLTs assessed in this analysis have all demonstrated significant reductions in CV events in large, randomized, placebo-controlled trials [9, 23, 25,26,27].
This study also observed key trends in relation to the real-world use of LDL-C testing. Over 5% of patients did not have an LDL-C test in the year preceding their AMI date. Further, approximately 25% of patients were excluded from the study cohort due to a lack of LDL-C testing available within 1 year of their LLT intensification. Post-AMI, most individuals on statins were found to initiate statin treatment without having an LDL-C test following their AMI (possibly due to initiation of statins occurring on or shortly after AMI), while most PCSK9i patients had an LDL-C test before treatment initiation. More frequent monitoring of PSCK9i patients before treatment could be to ascertain if PCKS9i intensification is required, or due to payer requirements for PCSK9i initiation. Given the time to initiation of PCKS9i observed in our study, earlier intensification to PCKS9i could result in earlier demonstrated reductions of LDL-C and CV events in high-risk patients.
The current study provides a large population-based sample of contemporary real-world Canadian data. The opportunity to link multiple administrative health databases in Alberta increased the validity and generalizability of the study. Moreover, we were able to identify a high-risk subgroup of patients with AMI, as per recent Canadian guidelines, and assess the impact of various LLTs on this population. To our knowledge, this is the first analysis of this type to be done.
There were several methodological limitations. LDL-C testing was reliant on usual care and as a result was often not reported before and after LLT initiation for all patients. Further, timing for the LDL-C testing varied and may not be reflective of the true LDL-C levels immediately before initiating the LLT (based on our hierarchical classification). Although ApoB and Lp(a) are now recognized in the new guidelines as important lipoprotein markers of CV risk, [3] at the time of this analysis there was limited data available, therefore, were not included in these analyses. Lastly, the PCSK9i monoclonal antibodies evolocumab and alirocumab were first available in 2015 for use in Canada with CV outcomes trials published in 2017/18, [9, 27] and while uptake has steadily increased with improved access since that time, the inclusion/exclusion criteria and the timeframe of our study likely limited the sample size for this treatment category.
Future research in this area may be required; in particular, the economic burden of the lag time between ASCVD events and PCSK9i treatment initiation (and potential for subsequent CV events) is required to better quantify the benefit of PCSK9i treatment.
Conclusions
Canadian and international guidelines recommend the intensification of statin treatment with ezetimibe and/or PCKS9i in patients who are unable to achieve LDL-C thresholds with statins alone, particularly in high-risk patients. This study provides further support that in a real-world Canadian population of very high-risk AMI patients, PCSK9i treatment in combination with other LLT provides the greatest reductions in LDL-C and guideline threshold achievement.
Change history
08 March 2023
“The supplementary table and figure citations corrected.
15 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40119-023-00310-z
References
Chen G, Farris MS, Cowling T, Pinto L, Rogoza RM, MacKinnon E, et al. Prevalence of atherosclerotic cardiovascular disease and subsequent major adverse cardiovascular events in Alberta, Canada: a real-world evidence study. Clin Cardiol. 2021;44(11):1613–20.
Sarak B, Savu A, Kaul P, McAlister FA, Welsh RC, Yan AT, et al. Lipid testing, lipid-modifying therapy, and PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) inhibitor eligibility in 27 979 patients with incident acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2021;14(4): e006646.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–50.
Sud M, Han L, Koh M, Abdel-Qadir H, Austin Peter C, Farkouh Michael E, et al. Low-density lipoprotein cholesterol and adverse cardiovascular events after percutaneous coronary intervention. J Am Coll Cardiol. 2020;76(12):1440–50.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–143.
Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2022;80(14):1366–418.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41(1):111–88.
Gupta M, Mancini GBJ, Wani RJ, Ahooja V, Bergeron J, Manjoo P, et al. Real-world Insights into Evolocumab Use in Patients with Hyperlipidemia: Canadian Analysis from the ZERBINI Study. CJC Open.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Gaudet D, López-Sendón JL, Averna M, Bigot G, Banach M, Letierce A, et al. Safety and efficacy of alirocumab in a real-life setting: the ODYSSEY APPRISE study. Eur J Prev Cardiol. 2022;28(17):1864–72.
Tu JV, Khan AM, Ng K, Chu A. Recent temporal changes in atherosclerotic cardiovascular diseases in Ontario: clinical and health systems impact. Can J Cardiol. 2017;33(3):378–84.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Halfon P, Eggli Y, van Melle G, Chevalier J, Wasserfallen JB, Burnand B. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–87.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Cannon CP, De Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6(9):1060.
Derington CG, Colantonio LD, Herrick JS, Cook J, King JB, Rosenson RS, et al. Factors Associated With PCSK9 Inhibitor Initiation Among US Veterans. Journal of the American Heart Association. 2021;10(8).
Grégoire J, Champsi S, Jobin M, Martinez L, Urbich M, Rogoza RM. Cost-Effectiveness Analysis of Evolocumab in Adult Patients with Atherosclerotic Cardiovascular Disease in Canada. Advances in Therapy. 2022.
Ray KK, Dhalwani N, Sibartie M, Bridges I, Ebenbichler C, Perrone-Filardi P, et al. Low-density lipoprotein cholesterol levels exceed the recommended European threshold for PCSK9i initiation: lessons from the HEYMANS study. Euro Heart J—Quality Care Clin Outcomes. 2022;8(4):447–60.
McKinley EC, Bittner VA, Brown TM, Chen L, Colantonio LD, Exter J, et al. Factors associated with time to initiation of a PCSK9 inhibitor after hospital discharge for acute myocardial infarction. J Clin Lipidol. 2022;16(1):75–82.
Gencer B, Mach F, Murphy SA, De Ferrari GM, Huber K, Lewis BS, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction. JAMA Cardiol. 2020;5(8):952.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962–71.
Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT trial. J Am Coll Cardiol. 2016;67(4):353–61.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Acknowledgements
This study is based on data provided by Alberta Health and Alberta Precision Laboratories. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Precision Laboratories. Neither the Government of Alberta nor Alberta Health expresses any opinion in relation to this study.
Funding
This study was supported and funded by Amgen Canada Inc. (Amgen), Mississauga, Ontario. Amgen collaborated with Medlior Health Outcomes Research Ltd. (Medlior) in the study design of the project. Medlior was responsible for requesting and analyzing the data, as well as reporting of the results. Amgen is also funding the publication of this study including Cardiology and Therapy’s Rapid Service Fee.
Author contributions
All authors Erin S. Mackinnon, Bryan Har, Salimah Champsi, Rajvi J. Wani, Lee Geyer, Eileen Shaw, Megan S. Farris and Todd J. Anderson contributed to the study conception and design. Material preparation and data analysis were performed by Lee Geyer. The first draft of the manuscript was written by Eileen Shaw, Erin S. Mackinnon, Salimah Champsi and Rajvi J. Wani and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Lee Geyer, Megan S. Farris, and Eileen Shaw are employed by Medlior, which received funding for the study from Amgen Canada. Erin S. Mackinnon, Salimah Champsi and Rajvi J. Wani are employed by Amgen Canada Inc. who funded this study and hold Amgen stock. Todd J. Anderson was the local Principal Investigator for research studies sponsored by Novartis in the past two years. Bryan Har was the local Principal Investigator for research studies sponsored by CSL Behring, Bristol Myers Squibb, and Idorsia.
Compliance with ethics guidelines
The study complies with the Declaration of Helsinki and was approved by the Health Research Ethics Board of Alberta—Community Health Committee (HREBA-CHC).
Data availability
Data was obtained via data request to Alberta Health. Data from this study are not publicly available and cannot be shared due to privacy reasons and ethical restrictions, as per the research agreement with Alberta Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised: The revised version of supplementary material updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mackinnon, E.S., Har, B., Champsi, S. et al. Guideline LDL-C Threshold Achievement in Acute Myocardial Infarction Patients: A Real-World Evidence Study Demonstrating the Impact of Treatment Intensification with PCSK9i. Cardiol Ther 12, 327–338 (2023). https://doi.org/10.1007/s40119-022-00300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00300-7