Abstract
Introduction
The 2021 Canadian Cardiovascular Society (CCS) guidelines recommend intensive low-density lipoprotein cholesterol (LDL-C) reduction for patients with atherosclerotic cardiovascular disease (ASCVD). For patients above LDL-C threshold on maximally tolerated statins, adding ezetimibe and/or a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) is recommended. This population-based, real-world study examined cardiovascular (CV) events in patients with ASCVD who are on statins and above current guideline threshold LDL-C levels.
Methods
Using administrative health data in Alberta, Canada, we identified patients with myocardial infarction (MI), ischemic stroke (IS), or peripheral artery disease with LDL-C > 1.8 mmol/L on statins between April 1, 2010 and March 31, 2016. Exploratory subgroups included very high-risk patients with ASCVD shown to derive the most benefit from PCSK9i intensification as identified by the CCS guidelines, including those with acute coronary syndrome (ACS) or recent MI. Frequencies and rates of individual and composite CV events (primary outcome: MI, IS, hospitalization for unstable angina, coronary revascularization, cardiovascular death; secondary outcome: MI, IS, CV death) were calculated over follow-up.
Results
The study included 32,984 patients with a mean (standard deviation) follow-up of 40.8 (21.0) months. Overall, 17.7% and 15.6% experienced a primary and secondary outcome, respectively, with rates of 5.58 and 4.83 per 100 patient-years, respectively. CV death and MI were the most common events. Subgroups with recurrent MI and comorbid diabetes exhibited higher CV event rates (23.6% and 22.2% had a primary outcome, respectively). Rates of CV events were notably high in patients with ACS or recent MI (49.4% and 54.0% had a primary outcome, respectively).
Conclusion
This real-world study confirms that statin-treated high-risk patients with ASCVD and above-threshold LDL-C levels have substantial incidence of recurrent CV events. These findings reinforce the opportunity for lipid-lowering therapy intensification in high-risk patients to levels below guideline-recommended threshold in order to reduce CV risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
Patients with atherosclerotic cardiovascular disease (ASCVD) have high morbidity and mortality, and a continued focus on developing effective prevention and management strategies to mitigate its impact on patients is required. | |
The purpose of this study was to quantify the risk of cardiovascular (CV) events in real-world, patients with stable ASCVD and low-density lipoprotein cholesterol (LDL-C) levels above the guideline-recommended threshold, despite statin therapy. | |
What was learned from the study? | |
The study identified that patients with stable ASCVD and LDL-C levels above currently recommended thresholds on statin therapy have a high risk of CV outcomes, with CV death and myocardial infarction being the most frequent CV events. | |
The study reiterates the need for intensifying lipid-lowering therapy, particularly in very high-risk subgroups, to reduce the risk of CV events in patients with stable ASCVD and LDL-C levels above guideline-recommended threshold on statin therapy. |
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains a leading cause of morbidity and mortality in Canada, despite diagnostic and treatment advances [1,2,, 2]. In the province of Alberta, Canada, the 5-year prevalence of ASCVD was recently estimated at 8.99% (or 89.9 per 1000 persons), highlighting the disease’s significant burden on the population [1]. The persistence of ASCVD as a leading cause of morbidity and mortality necessitates a continued focus on developing effective prevention and management strategies to mitigate its impact on patients.
The most recent Canadian Cardiovascular Society (CCS) guidelines for the management of dyslipidemia (2021) highlight the importance of intensively lowering low-density lipoprotein cholesterol (LDL-C) in patients with ASCVD to decrease the risk of cardiovascular (CV) events [3]. Further, in patients treated with the maximally tolerated dose of statin and LDL-C level above the threshold of 1.8 mmol/L, the addition of non-statin lipid-lowering therapies (LLTs) is recommended. For patients with LDL-C levels between 1.8 and 2.2 mmol/L, the recommended addition to statin treatment is ezetimibe and/or a protein convertase subtilisin/kexin type 9 serine protease inhibitor (PCSK9i), while in patients with LDL-C levels above 2.2 mmol/L, PCSK9i are recommended with or without ezetimibe. The CCS guidelines additionally identify high-risk patients shown to derive the largest absolute benefit from LLT intensification with a PCSK9i. They include patients with acute coronary syndrome (ACS), diabetes mellitus, recurrent myocardial infarction (MI), MI in the past 2 years, and LDL-C of 2.6 mmol/L or above, among others [3].
Clinical trial evidence from the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) [4] and ODYSSEY OUTCOMES [5] studies have demonstrated the reduced risk of CV outcomes with PCSK9i added to statin therapy in patients with ASCVD. Subgroup analyses from both trials have identified greater absolute risk reduction in CV events in patients with very high baseline CV risk, such as those with recent or recurrent MI, prior coronary artery bypass graft surgery, and diabetes [6,7,8,9,10]. However, as few studies have investigated CV outcomes in real-world diverse populations of high- and very high-risk patients with ASCVD treated with statins who remain above the LDL-C threshold of 1.8 mmol/L [11, 12], the objective of this study was to estimate the real-world rate and frequency of CV events in this population. Additionally, an exploratory objective aimed to further assess CV events among several of the very high-risk ASCVD subgroups identified by the CCS guidelines.
Methods
Study Design and Population
A retrospective observational cohort study was conducted using administrative health data in Alberta, Canada. The study population included adult Albertan residents who met the FOURIER trial inclusion and exclusion criteria [4] between April 1, 2010 and March 31, 2016 (Fig. 1; case ascertainment period) with at least 1 year of pre-index and 1 year of follow-up data. Within the case ascertainment period, patients aged 40–85, with MI, ischemic stroke (IS), or peripheral arterial disease (PAD) were indexed on April 1, 2011 or every 2.5 years after (October 1, 2013 or April 1, 2016), based on when inclusion criteria were met. Patients then had variable follow-up periods between April 1, 2011 and March 31, 2018 to evaluate CV events of interest. Included patients required an LDL-C test of at least 1.8 mmol/L or non-HDL-C test of at least 2.6 mmol/L prior to the index date while on moderate/high intensity statin therapy (see Supplementary Material Table 1 for the full inclusion criteria including definition of risk factors). Patients were excluded [4] if they experienced an MI or IS within 4 weeks of their index date, had a known history of hemorrhagic stroke (defined previously [11, 13]), had previously received any major organ transplant, or had a prescription dispensation for a PCSK9i prior to their index date. Patients were censored from follow-up at the point of receiving a PCSK9i prescription dispensation if this occurred during the follow-up period.
Exploratory Subgroups
Subgroups of interest were pre-defined for the exploratory objective, focusing on patients with ASCVD considered to be very high CV risk as defined in the 2021 CCS guidelines [3]. This included patients with diabetes mellitus, recurrent MI, MI in the past 2 years, and LDL-C of 2.6 mmol/L or above (most recent test prior to index date) despite stain therapy (Supplementary Material Table 2). The very high-risk subgroups of ACS and recent MI were considered separately as they were defined as patients who had an ACS event (MI or unstable angina [UA]) during the follow-up period and were re-indexed on this date to assess additional CV outcomes; the patients with recent MI were a subset of the ACS subgroup.
Data Sources
The study utilized the following datasets from Alberta Health and Alberta Precision Laboratories: (1) National Ambulatory Care Reporting System (NACRS), including facility-based ambulatory care information on diagnostic and procedure codes (e.g., emergency department [ED] visits); (2) Discharge Abstract Database (DAD), including inpatient hospitalizations and diagnostic and procedure intervention codes; (3) Alberta Precision Laboratories dataset, including laboratory tests and results; (4) Population Registry, including demographic and geographic data; (5) Pharmaceutical Information Network (PIN) dataset, including prescription dispense information regardless of payer; (6) Practitioner Claims, including provider claims data for insured health services; and (7) Vital Statistics, reporting date and cause of death information.
The study complies with the Declaration of Helsinki and was approved by the Health Research Ethics Board of Alberta–Community Health Committee (HREBA-CHC). Patient consent for participation was waived.
Study Variables
Patient characteristics including age and sex at index date and baseline LLTs (statins/ezetimibe) within the 12 months prior were assessed. Lipid values at baseline were the most recent test prior to index date and within 12 months. Clinical history was derived on the basis of diagnosis and procedure codes for MI, IS, PAD, diabetes, non-MI related coronary revascularization procedure, coronary artery disease, and metabolic syndrome prior to index date (as defined in Supplementary Material Table 1). CV outcomes (individual and composite) following index date were examined using inpatient hospitalizations only, except for CV death, which was extracted from inpatient deaths, ED deaths, and Vital Statistics. The average follow-up time was calculated. Individual CV outcomes included MI, IS, hospitalization for UA, coronary revascularization, and CV death as defined in Supplementary Material Table 2. Composite CV outcomes were categorized into primary (MI, IS, hospitalization for UA, coronary revascularization, and CV death) and secondary (MI, IS, and CV death).
Statistical Methods
Patient characteristics were summarized descriptively. CV outcomes within the follow-up period were described as frequencies, proportions, and rates (per 100 patient-years with 95% confidence intervals [CI]) for first individual CV events and the primary and secondary composite outcomes. Composite outcome rates were based on the first outcome of any type within the composite category for each patient.
The total number and rates of all CV events (per 100 patient-years) during the follow-up period were also calculated for individual outcomes (i.e., the count of each type of event following index) and composite outcomes (the count of all types of events following index). Events of the same type required a minimum of 30 days between events to be considered as two separate events. A subsequent CV event of a different type was counted as a new event even if it occurred within 30 days of the previous event. If the subsequent event was a revascularization procedure, a minimum of 30 days must have passed for the second revascularization procedure to be considered a separate event regardless of the type of the first CV event.
Similar analyses were conducted for the exploratory subgroups, which were not mutually exclusive. For the ACS and recent MI subgroups, patients were re-indexed on the first ACS event (MI or UA) date during the follow-up period and followed from the re-index date to estimate additional CV outcome rates.
All statistical analyses, including data cleaning, linkage, cohort derivation and analysis, were conducted in SAS 9.4®.
Results
Study Cohort and Patient Characteristics
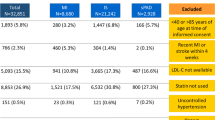
The study cohort consisted of 32,984 patients with a mean (standard deviation [SD]) follow-up time of 40.8 (21.0) months, approximately 3.4 years (Fig. 2). The mean (SD) age was 68.2 (10.5) years and 34.4% were female (Table 1). At index date, 60.0% (n = 19,801) of patients were on a high-intensity statin, while 40.0% (n = 13,183) were on a moderate-intensity statin. Only 6.0% (n = 1968) of patients received ezetimibe. No patients were excluded as a result of PCSK9i use prior to index date, and less than 1% (n = 38) of patients were prescribed a PCSK9i during follow-up (data not shown; patients were censored from follow-up at time of PCSK9i initiation). The mean (SD) LDL-C level of the study cohort was 2.08 (0.94) mmol/L (median (interquartile range, IQR) 1.88 (1.42–2.55) mmol/L). While qualifying events described are not mutually exclusive, MI was the most common qualifying event in the study cohort (54.7%), and among these, 26.8% of MI occurred within 6 months of index date. Stroke was the second most common at 40.3%; 25.7% of these occurred within 6 months of index date. Diabetes was prevalent at 43.9% as was history of non-MI-related coronary revascularization procedure (42.0%).
Cohort flow diagram. ACS acute coronary syndrome, AH Alberta Health, ASCVD atherosclerotic cardiovascular disease, CABG coronary artery bypass graft, FOURIER further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk, IS ischemic stroke, LDL-C low-density lipoprotein cholesterol, MI myocardial infarction, PAD peripheral arterial disease, PCSK9i proprotein convertase subtilisin/kexin type 9 serine protease inhibitors, UA unstable angina. aSubgroups are not mutually exclusive. bRecent MI is a subgroup of the ACS group
CV Outcomes in the Study Cohort
Over the follow-up period, 17.7% (n = 5836) of patients had a first primary and 15.6% (n = 5143) had a first secondary outcome, which corresponded to incidence rates (95% CI) of 5.58 (5.44–5.73) and 4.83 (4.69–4.96) per 100 patient-years, respectively (Fig. 3). The total CV event rates (95% CI) over the follow-up period for primary and secondary outcomes were 8.63 (8.46–8.81) and 6.58 (6.43–6.73) per 100 patient-years, respectively. The most frequently identified individual types of CV events were CV death (n = 2862; 8.7%) and MI (n = 2185; 6.6%), corresponding to rates (95% CI) of 2.56 (2.46–2.65) and 2.02 (1.94–2.11) per 100 patient-years, respectively (Supplementary Material Table 3). When considering total individual CV events over follow-up, the highest rate (95% CI) was observed for MI at 2.80 (2.70–2.90) per 100 patient-years.
Rates per 100 patient years of first and total CV events during follow-up for the overall cohort. CV cardiovascular, IR incidence rate, IS ischemic stroke, MI myocardial infarction, aPrimary outcome was defined as CV death, MI, IS, hospitalization for unstable angina, or coronary revascularization. bSecondary outcome was defined as CV death, MI, IS. IRs are written as IR (95% confidence interval)
Exploratory Analysis of Very-High Risk ASCVD Subgroups
The very high-risk subgroups of interest included diabetes mellitus (n = 13,415), recurrent MI (n = 9603), MI in past 2 years (n = 14,709), and patients with the most recent LDL-C test result prior to index date of 2.6 mmol/L or above (n = 7780). At index date, the mean age was comparable across high-risk ASCVD subgroups of interest (Table 2). A higher proportion of patients in the recurrent MI (79.3%) and MI in the past 2 years (78.8%) subgroups were observed to be on high-intensity statins compared to the overall cohort (Table 1), although no statistical testing was performed.
In Fig. 4, the rates of primary and secondary outcomes for subgroups of patients with MI in the past 2 years or LDL-C of 2.6 mmol/L or above were similar to the overall cohort (Fig. 3), whereas, for patients with comorbid diabetes or recurrent MI, rates were higher (primary outcome (95% CI) 7.40 (7.14–7.67) and 6.83 (6.55–7.11) per 100 person-years, respectively; secondary outcome (95% CI 6.51 (6.26–6.76) and 5.62 (5.37–5.87) per 100 person-years, respectively). The total primary outcomes over follow-up reflected a similar pattern. When assessing the individual CV events, MI and CV death rates were the highest (Supplementary Material Table 4). In addition, the highest rates of coronary revascularization were in patients with recurrent MI (rate (95% CI) 2.11 (1.96–2.26) per 100 patient-years for total events over follow-up).
Rates per 100 patient years of CV events for the very high-risk subgroups. ASCVD atherosclerotic cardiovascular disease, CV cardiovascular, IR incidence rate, IS ischemic stroke, LDL-C low-density lipoprotein cholesterol, MI myocardial infarction. aIncludes type 1 and type 2 diabetes. bPrimary outcome was defined as CV death, MI, IS, hospitalization for unstable angina, or coronary revascularization. cSecondary outcome was defined as CV death, MI, IS. IRs are written as IR (95% confidence interval)
Exploratory Analysis of Very-High Risk ACS and Recent MI Subgroups
The additional very high-risk subgroups based on MI or UA events occurring during the follow-up period included patients with ACS (n = 2691) and recent MI (n = 2185). Patient characteristics of the ACS and recent MI subgroups at baseline were similar to the overall study cohort (Table 3). However, ezetimibe use was slightly higher in these subgroups compared to the overall cohort (9.1% for ACS, 8.7% for recent MI vs. 6.0%). In the ACS subgroup, 49.4% of patients experienced an additional primary outcome following the re-index date, corresponding to a rate (95% CI) of 38.41 (36.40–40.53) per 100 patient-years, and 44.4% experienced an additional secondary outcome (rate of 31.01 [29.30–32.81] per 100 patient-years) (Fig. 5).The highest proportions and rates of CV events were observed in the recent MI subgroup, where 54.0% of patients experienced an additional primary outcome following the re-index date, with a rate (95% CI) of 47.73 (45.08–50.53) per 100 patient-years. The additional secondary outcome (95% CI) was also high at 43.27 (40.82–45.86) per 100 patient years.
Rates per 100 patient years of additional CV events for the very high-risk sub-groups: ACS and recent MI. ACS acute coronary syndrome, CV cardiovascular, IR incidence rate, IS ischemic stroke, MI myocardial infarction. aPrimary outcome was defined as CV death, MI, IS, hospitalization for unstable angina, or coronary revascularization. bSecondary outcome was defined as CV death, MI, IS. IRs are written as IR (95% confidence interval)
When total additional CV outcomes were considered in the ACS and recent MI subgroups, there were high primary outcome rates (95% CI) of 47.62 (45.77–49.55) and 56.90 (54.57–59.33), and secondary outcome rates (95% CI) of 35.13 (33.54–36.79) and 44.75 (42.69–46.91) per 100 patient-years, respectively. The additional individual CV events are described in Supplementary Material Table 5, where total rates of MI and CV death were the highest individual reported outcomes over the study period (total individual events).
Discussion
Our study investigated a Canadian cohort of patients with ASCVD and a history of MI, IS, or PAD, and LDL-C above the current recommended threshold on moderate-high intensity statin therapy, making them high-risk for subsequent CV events. Patients had a mean LDL-C value of 2.08 mmol/L, which is also above the previous guidelines’ goal of less than 2.0 mmol/L [14,15,16], and above the current recommended threshold of 1.8 mmol/L [3]. Results demonstrated that CV outcome rates were high for this cohort of patients with ASCVD, and patients continued to experience high total CV outcome rates during the follow-up period. Exploratory subgroup analyses of very high-risk patients with ASCVD identified in the recent CCS guidelines [3] revealed higher CV event rates in patients with recurrent MI and comorbid diabetes compared to the overall cohort. Furthermore, patients with ACS and recent MI had very high rates of additional CV outcomes over time, reinforcing the very high risk of recurrent events. MI and CV death were the most common individual CV events over follow-up, in the overall population and across all very high-risk subgroups. Our findings highlight the sobering high morbidity and mortality rates for patients with ASCVD who remain above recommended LDL-C threshold, despite statin treatment.
The evidence linking reduction in LDL-C to subsequent reduction in CV outcomes has increased substantially over time [17], leading to global adoption of guidelines further lowering recommended thresholds/targets of LDL-C in patients with ASCVD [18, 19]. The lower LDL-C threshold for the use of additional lipid lowering drugs of 1.8 mmol/L has recently been recommended by the CCS to reduce CV risk in patients with ASCVD already receiving maximally tolerated statin [20]. Evidence contributing to this recommendation includes CV outcome clinical trials with non-statin therapies such as ezetimibe (IMPROVE-IT) [21] and PCSK9is (FOURIER [4, 6, 22] and ODYSSEY OUTCOMES [5, 23]). These trials demonstrated the CV benefit of non-statin therapies when added to statin in secondary prevention patients compared to placebo-treated patients. In the FOURIER trial, whose patient characteristics are emulated in our study, the addition of evolocumab to statin therapy resulted in a rapid and sustained reduction in LDL-C down to levels lower than previously reported in other lipid-lowering trials (median achieved LDL-C 0.78 mmol/L). Event rates were 9.8% and 11.3% in the evolocumab and placebo-treated groups, respectively, for the primary endpoint (a composite of CV death, MI, stroke, hospitalization for UA, or coronary revascularization), resulting in significant reduction in CV events (20% relative risk reduction in the composite of CV death, MI and stroke; p < 0.001) [4]. A linear relationship between achieved LDL-C and reduction in CV events was also established [24]. Long-term follow-up with PCSK9i treatment has reinforced this concept [25]. This current analysis captures a similar patient population to the FOURIER study and demonstrates, in a real-world population, the high morbidity and mortality that occurs in these high-risk patients in an era where PCSK9i treatment was not available.
Despite this growing body of evidence, a large care gap still exists in Canada. A recent analysis in Alberta found that 53% of patients with ASCVD and 30% of patients with acute MI were not treated with a statin; less than 3% were treated with ezetimibe [1]. In a population-based cohort of post-percutaneous coronary intervention (PCI) patients in Ontario, over 40% of patients with an LDL-C test remained above 1.8 mmol/L 6 months post-PCI [12]. This was correlated with a significantly higher risk of CV outcomes, which increased the further away from the threshold that patients were [12]. Further, in an analysis of very high-risk patients with recent ACS in Alberta, approximately 80% of patients had an LDL-C above 1.8 mmol/L or non-HDL-C above 2.6 mmol/L within 90 days post-discharge [26]. The authors determined that approximately 40% of the patients assessed in this real-world cohort would have been eligible for treatment intensification with PCSK9i, which could have reduced their risk of further CV events [26]. Indeed, a recent analysis in a post-MI population found that the proportion of patients achieving below the 1.8 mmol/L threshold increased by 77.7% in patients treated with statins and PCSK9i in combination after their MI [27]. This was substantially higher than in patients treated with ezetimibe and statin (45.4%) or with high-intensity statin alone (32.4%) [27]. Taken together, these results reiterate the urgent need to further reduce residual LDL-C-related risk in Canadian patients with ASCVD and support clinical guidelines [3, 18, 19] for therapeutic intensification with non-statin therapies in patients who are unable to attain LDL-C recommended thresholds on statin therapy alone.
In our study, of patients with LDL-C or non-HDL-C level above guideline-recommended thresholds, despite being on moderate to high-intensity statins, ezetimibe use was very low. As a result of the inclusion criteria, the median LDL-C was 1.88 (IQR 1.42–2.55) mmol/L in this cohort and approximately half of these patients had an LDL-C above the currently recommended 1.8 mmol/L. We demonstrated that over the course of follow-up (mean of 41 months), approximately 1 in 5 patients experienced a CV event and nearly 1 in 10 died. This risk is amplified among the very high-risk subgroups in this cohort; patients with ACS and recent MI were identified as having the highest risk of recurrent CV events, with both higher proportions and rates of additional events over follow-up. Notably, approximately half of patients with ACS and recent MI experienced additional CV events, while approximately one-quarter of patients experienced CV death. These results highlight that patients with ACS and recent MI require swift and intensive LDL-C intervention to reduce CV morbidity and mortality. The addition of evidence-based non-statin therapies in line with current Canadian guideline recommendations has the potential to decrease the impact of this care gap. Indeed, this significant reduction in events has been demonstrated with PCSK9is in ODYSSEY OUTCOMES (a 1–12-month post-ACS population) and in a pre-specified analysis of patients with recent MI (< 12 months) in FOURIER [5, 6]. Future research should focus on better understanding how to optimize non-statin therapy use in very high-risk patients with ASCVD, particularly those with ACS and recent MI.
Our study had the strength of examining a large, trial-like population of patients identified with ASCVD in Alberta, Canada; however, some limitations should be considered. First, administrative data are not collected specifically for research; rather, they are for billing, monitoring, and hospital administrative purposes. This may have introduced potential misclassification bias of the study cohort and CV outcomes due to incorrect ICD coding and lack of independent adjudication. CV events were assessed on the basis of inpatient or ED deaths, and Vital Statistics, but as a result of the lag time in the Vital Statistics dataset for out-of-hospital death, rates of CV deaths at the end of our study period may be underestimated. Additionally, administrative health data do not include certain patient and clinical characteristics such as lifestyle factors and behaviors (e.g., smoking), which can impact CV event rates. There are other residual risk factors for CV outcomes including other lipoproteins (i.e., lipoprotein(a), triglycerides), inflammatory risk factors, and smoking, which were not assessed in this analysis. Testing of lipids was not done in a central laboratory, which could lead to variability. Furthermore, the use of a fixed index date could potentially exclude patients who died prior to the three fixed index dates, leading to a reduced sample size and potential selection bias. Another limitation is that the study focused on LDL-C/non-HDL-C levels at baseline; these were not examined over time. Our study also did not track LLTs over time, only at baseline, where the potential of non-adherence or removal of statin/ezetimibe therapy could have inflated incidence rates. Statins could also have been uptitrated, or ezetimibe added, which may have reduced CV outcome rates over time. Finally, other secondary prevention medications were not captured at baseline or during follow-up.
Conclusion
Results from our study provide important real-world evidence of the high rates of CV outcomes among patients with stable ASCVD who remain above guideline-recommended LDL-C thresholds despite statin therapy in Alberta, Canada. The high incidence of CV events, particularly MI and CV death, underscores the urgent need to reduce LDL-C levels below guideline thresholds to reduce morbidity and mortality, especially in patients with ACS or recent MI. These findings highlight the importance of implementing the newest CCS guideline recommendations to optimize non-statin therapy to reduce the risk of CV outcomes in this patient population.
Data Availability
Data were obtained through a data request to Alberta Health. Data from this study are not publicly available and cannot be shared due to privacy reasons and ethical restrictions, as per the research agreement with Alberta Health.
References
Chen G, Farris MS, Cowling T, et al. Prevalence of atherosclerotic cardiovascular disease and subsequent major adverse cardiovascular events in Alberta, Canada: a real-world evidence study. Clin Cardiol. 2021;44(11):1613–20.
Tu JV, Khan AM, Ng K, Chu A. Recent temporal changes in atherosclerotic cardiovascular diseases in Ontario: clinical and health systems impact. Can J Cardiol. 2017;33(3):378–84.
Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–50.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Gencer B, Mach F, Murphy SA, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol. 2020;5(8):952–7.
Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–50.
Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from FOURIER. Circulation. 2018;138(8):756–66.
Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–28.
Goodman SG, Aylward PE, Szarek M, et al. Effects of alirocumab on cardiovascular events after coronary bypass surgery. J Am Coll Cardiol. 2019;74(9):1177–86.
Lindh M, Banefelt J, Fox KM, et al. Cardiovascular event rates in a high atherosclerotic cardiovascular disease risk population: estimates from Swedish population-based register data. Eur Heart J Qual Care Clin Outcomes. 2019;5(3):225–32.
Sud M, Han L, Koh M, et al. Low-density lipoprotein cholesterol and adverse cardiovascular events after percutaneous coronary intervention. J Am Coll Cardiol. 2020;76(12):1440–50.
Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–81.
Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263–82.
Anderson TJ, Grégoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151–67.
Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009;25(10):567–79.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–143.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41(1):111–88.
Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Murphy SA, Pedersen TR, Gaciong ZA, et al. Effect of the PCSK9 inhibitor evolocumab on total cardiovascular events in patients with cardiovascular disease: a prespecified analysis from the FOURIER trial. JAMA Cardiol. 2019;4(7):613–9.
Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682–9.
Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962–71.
Gaba P, O’Donoghue ML, Park JG, et al. Association between achieved low-density lipoprotein cholesterol levels and long-term cardiovascular and safety outcomes: an analysis of FOURIER-OLE. Circulation. 2023;147(16):1192–203.
Sarak B, Savu A, Kaul P, et al. Lipid testing, lipid-modifying therapy, and PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor eligibility in 27 979 patients with incident acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2021;14(4):e006646.
Mackinnon ES, Har B, Champsi S, et al. Guideline LDL-C threshold achievement in acute myocardial infarction patients: a real-world evidence study demonstrating the impact of treatment intensification with PCSK9i. Cardiol Ther. 2023;12:327–38.
Acknowledgements
This study is based on data provided by Alberta Health and Alberta Precision Laboratories. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study. We would also like to thank the following from Medlior Health Outcomes Research Ltd. (Medlior): Ransi Nayakarathna and Suzanne McMullen for their support in the study start-up, Guanmin Chen and John Paul Ekwaru for their support in data analyses.
Medical Writing/Editorial Assistance.
We would like to thank Heather Neilson for her editorial assistance, who is an employee of Medlior Health Outcomes Research Ltd. This editorial assistance was funded by Amgen Canada.
Funding
This work and the associated journal publication fees were supported by Amgen Canada Inc. (Amgen), Mississauga, Ontario, Canada. Amgen collaborated with Medlior in the study design of the project. Medlior was responsible for the request and statistical analysis of the data, as well as reporting of the results.
Author information
Authors and Affiliations
Contributions
All authors (Erin S. Mackinnon, Lawrence A. Leiter, Rajvi J. Wani, Natasha Burke, Eileen Shaw, Kelcie Witges, and Shaun G. Goodman) contributed to the study conception and design. Erin S. Mackinnon, Rajvi J. Wani, Natasha Burke, Eileen Shaw and Kelcie Witges drafted the work and all authors revised it critically and have approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
This study was sponsored by Amgen Canada Inc. (Amgen). Erin S. Mackinnon, Rajvi J. Wani, and Natasha Burke are employed by Amgen, who funded this study and hold Amgen stock. Lawrence A. Leiter has received research support from, has provided CME on behalf of and/or has acted as an advisor to Amarin, Amgen, AstraZeneca, Esperion, HLS, Kowa, Merck, Novartis, Pfizer, and Sanofi. Eileen Shaw and Kelcie Witges are employed by Medlior Health Outcomes Research Ltd., which received funding for the study from Amgen. Shaun G. Goodman reports research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, CYTE Ltd., Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Tolmar Pharmaceuticals, Valeo Pharma; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Failure Society, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, PERFUSE Research Institute, TIMI Study Group (Brigham Health).
Ethical Approval
The study complies with the Declaration of Helsinki and was approved by the Health Research Ethics Board of Alberta–Community Health Committee (HREBA-CHC). Patient consent for participation was waived.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mackinnon, E.S., Leiter, L.A., Wani, R.J. et al. Real-World Risk of Recurrent Cardiovascular Events in Atherosclerotic Cardiovascular Disease Patients with LDL-C Above Guideline-Recommended Threshold: A Retrospective Observational Study. Cardiol Ther 13, 205–220 (2024). https://doi.org/10.1007/s40119-024-00349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-024-00349-6