Abstract
Introduction
Patients with spontaneous sub-arachnoid hemorrhage (SAH) might develop various cardiac abnormalities, however; the prognostic implications of these cardiac abnormalities are not well known. This study aimed to detect the cardiac abnormality that correlates best with in-hospital all-cause mortality in patients with SAH.
Methods
In this retrospective study, all patients admitted to our institution with a primary diagnosis of SAH, and underwent a transthoracic echocardiogram (TTE) from July 2011 until May 2014, were enrolled. Data gathered included patients’ demographics, Hunt and Hess clinical grading, computed tomography SAH Fisher grading, troponin T level, electrocardiographic (ECG) changes, TTE, and in-hospital all-cause mortality. Multivariate logistic regression of the cardiac abnormalities and in-hospital all-cause mortality was performed.
Results
A total of 247 patients were included in our analysis. In-hospital all-cause mortality was 15.6% (38 patients). The presence of elevated troponin T levels, resting segmental wall motion abnormalities, reduced ejection fraction (<35%), and prolonged corrected QT interval (QTc) on ECG were associated with increased in-hospital all-cause mortality on univariate analysis. On multivariable regression, QTc prolongation was the only independent predictor for in-hospital all-cause mortality (p = 0.04).
Conclusions
Prolonged QTc interval on ECG was independently associated with in-hospital all-cause mortality in patients presenting with spontaneous SAH. Whether this is a causative association or a marker of underlying severe clinical presentation of SAH remains unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sub-arachnoid hemorrhage (SAH) is associated with a high mortality, particularly within the first 48 h of presentation [1, 2]. Although cardiac injury in SAH has been previously described [3,4,5], the prognostic implications of cardiac injury and its relationship with short-term mortality remain controversial. In a meta-analysis of 25 studies, there was a positive association between various cardiovascular abnormalities and mortality in SAH [6]. A few studies illustrated an association and prognostic significance of prolonged QTc in relation to SAH morbidity and mortality [7, 8], while other studies demonstrated that elevated troponin level was associated with increased mortality [9, 10]. A recent study suggested that resting segmental wall motion abnormality (RSWMA) was the only prognostic indicator of short-term mortality, while other cardiovascular abnormalities including QTc prolongation, elevated troponins, or pro-brain natriuretic peptide (BNP), were not significant on multivariable regression analysis [11]. The aim of our study was to further determine which cardiovascular abnormalities in patients with spontaneous SAH correlate with in-hospital all-cause mortality.
Methods
Study Population
All patients with SAH admitted to the University of Florida, a tertiary academic hospital, from July 2011 until May 2014, using International Statistical Classifications of Diseases (ICD)-9 code 430, for identification of SAH patients, were enrolled. As transthoracic echocardiogram (TTE) findings were pertinent to our study, only patients who had a TTE performed during the admission were included. TTE was identified by the Current Procedural Terminology (CPT) code 93303. Patients <18 years old, patients mislabeled as SAH, or those with traumatic SAH were excluded from the study. The diagnosis of spontaneous SAH was confirmed by reviewing the computerized tomography (CT) scan reports and the admission documentation notes.
The following data were obtained: patients’ age, sex, history of hypertension, diabetes, coronary artery disease, prior stroke, smoking status, chronic kidney disease, baseline Hunt-Hess score (from I to V) [12] and CT scan Fisher grading for SAH (from 1 to 4) [13]. Based on the heart rate measured on the first ECG report, we categorized the patients’ rhythm into: tachycardia (>100 bpm), normal (60–100 bpm), and bradycardia (<60 bpm). A prolonged QTc was defined as QTc interval >450 ms in males and >470 ms in females [14]. Bazett’s formula was used to calculate the QTc value from the report of first ECG performed after admission [15]. ST elevation was defined as elevation of >1 mm above the J-point in limb leads and 2 mm in chest leads. Ejection fraction was divided into: >55%, 35–55%, and <35%. The presence and location of RSWMA, apical ballooning, and global hypokinesis was also recorded. A cardiologist, expert in cardiac ultrasound, who was not directly involved in the study, reported the TTE results. Highly sensitive troponin T is the cardiac biomarker of choice in our institution, with a reference range of <0.03 ng/ml, thus 0.03 ng/ml and above was considered as elevated troponin T level. In-hospital mortality was confirmed by a death note recorded by the physician at the time of death. All study data were collected and managed using REDCap electronic data capture tools hosted at the University of Florida [16]. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. The study protocol was approved by the University of Florida Institutional Review Board, which has waived the informed consent given the retrospective nature of the study.
Statistical Analysis
Descriptive statistics was performed for the baseline demographics, ECG, TTE, troponin levels, and in-hospital mortality. Data were reported as frequencies for categorical variables, as well as mean and standard deviation for continuous variables. Logistic regression with robust standard errors [17] for the estimation of the odds ratio was performed to identify the significant predictors of in-hospital mortality. A final multivariate model included the significant predictors of in-hospital mortality. Results were reported as odds ratio (OR), and corresponding 95% confidence intervals (CI) for the cardiovascular abnormalities. Data analysis was conducted using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics
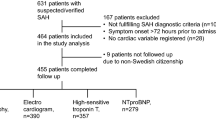
The study initially enrolled 2058 patients with SAH, 295 of them had a TTE performed during the hospitalization. Forty-nine patients had either traumatic SAH or were mislabeled as SAH and thus were excluded from the study. A total of 244 patients were included in the final analysis. The mean age was 59 years, with two-thirds of the patients being females. The majority of the patients had a clinical (Hunt–Hess) grading of II–IV and a CT Fisher grading of 2. In-hospital all-cause mortality was 15.6% of the patient population (Table 1). Compared to the SAH survivors, the deceased patients were older (p = 0.009), with a history of coronary artery disease (p = 0.02), higher Hunt-Hess grade (p < 0.001), and higher incidence of hydrocephalus (p = 0.021) (Table 2).
Cardiac Biomarkers, ECG, and TTE Findings
One hundred thirty-five patients had a troponin level drawn, 37% of those patients had an elevated troponin level during their admission (50 patients), with a mean peak troponin value of 0.15. Meanwhile, 193 patients had an ECG performed during their stay; 78% of those had an abnormal ECG finding (152 patients). The commonest ECG abnormality was prolonged QTc occurring in 57% (95 patients); 13% (26 patients) were found to have tachycardia. Sixteen percent of the total patient population (39 patients) had a RSWMA on TTE, and five patients were found to have findings compatible with takotsubo syndrome (Table 1).
Cardiovascular Predictors of in-Hospital All-Cause Mortality
On univariate analysis, prolonged QTc (p < 0.005), elevated troponin level (p = 0.03), EF < 35% (p = 0.03), and RSWMA (p = 0.03) were associated with an increased risk of in-hospital all-cause mortality. However, T-wave inversion (p = 0.12), ST elevation (p = 0.22), and tachycardia (p = 0.24) were not significantly associated with increased mortality (Table 3). On multivariable regression analysis, prolonged QTc was the only cardiac abnormality that was associated with increased in-hospital mortality (OR 5.1, 95% CI 1.04–25.28, p = 0.04) (Table 4).
Discussion
This retrospective analysis illustrates the prognostic significance of prolonged QTc, in relation to in-hospital all-cause mortality in patients with spontaneous SAH. The presence of prolonged QTc interval was associated with a five-times-increased risk of death in SAH patients, compared to those with normal QTc interval. This concurs with the previous studies that evaluated prolonged QTc interval prognostic significance [7, 8]. Ichinomiya et al. reached a conclusion similar to ours; however, they used the Glasgow Outcome Scale at hospital discharge, as a primary outcome, rather than in-hospital mortality. They found that a QTc of 448 ms at day 7 of admission was 73% sensitive (95% CI 68–78%) and 93% specific (95% CI 90–96%) prognostic tool to detect such outcome [7]. Marafioti et al. reported that prolonged QTc was an independent prognostic tool for detection of index mortality in SAH patients when it was compared to other prognostic risk factors such as clinical and CT grading of SAH. Most of the other cardiovascular abnormalities found in SAH such as T-wave inversion, ST changes, and RSWMA, were not reported in that analysis [8].
This study was not designed to address the pathophysiology behind prolonged QTc association with increased short-term all-cause mortality. However, the association might be explained by either a causal relationship, where prolonged QTc increases the chances of developing ventricular arrhythmias and thus sudden cardiac death [18], or the prolonged QTc could be just a reflection of severe SAH grade, which is associated with poor prognosis [7, 8]. A prospective study by Frontera et al. illustrated the prognostic significance of arrhythmia (including atrial fibrillation, atrial flutter, and sustained ventricular tachycardia) in SAH patients, showing an increased risk of mortality compared to those with normal heart rhythm (OR 8.0, 95% CI 1.9–34.0, p = 0.005) [19].
In a multicenter prospective study by van der Bilt, et al. [11], RSWMA was found to be the only prognostic indicator of short-term mortality, while other cardiovascular abnormalities including QTc prolongation, elevated troponins, or proBNP, lost their significance on multivariable regression analysis. Although our study reached a different conclusion, multiple considerations should be pointed out. First, that study primarily enrolled patients with mild clinical grading, and thus patients who might have the highest risk of in-hospital mortality were excluded. Also, it was not clear, from their method, how the QTc interval was analyzed and if it was incorporated in the multivariable regression analysis or not. Finally, their primary outcome was delayed cerebral ischemia while mortality at 3 months was reported as a secondary outcome, rather than index hospitalization mortality.
Elevated troponin level increased the risk of in-hospital death in our study by univariate analysis; however, this risk lost its significance after the multivariable regression analysis. This observation concurs with some of the previous studies [1, 11]. Meanwhile, studies reporting elevated troponin as a prognostic risk factor for mortality were not adjusted for other cardiovascular abnormalities [10, 20]. In addition, echocardiographic features of takotsubo cardiomyopathy have been linked to worse outcomes [21]; however, owing to the small number of patients who had findings of takotsubo syndrome (i.e., five patients), thus our study could not confirm this association.
Limitations of our study include: first, as a tertiary center we were more likely to have more complex patients, compared to non-tertiary hospital SAH population, however, no patients were excluded because of severity of SAH. Second, there were missing data regarding some of the cardiovascular abnormalities collected, including troponin levels and ECG reports; however, we do not believe that those were not large enough to affect our final analysis. Third, QTc interval was calculated by computer, which used Bazett’s formula. Bazett’s formula at best may be imprecise; however, it remains the simplest and most applicable formula for calculation of QTc [22]. Finally, the confidence interval for prolonged QTc association with in-hospital mortality is wide, which might be due to our sample size, and thus any conclusion drawn from our study needs to be confirmed in a larger patient population.
Conclusions
Among the variable cardiac abnormalities seen in patients with spontaneous SAH, prolonged QTc interval on ECG was the only independent cardiac predictor of in-hospital mortality. These data add to the current literature regarding the cardiac abnormalities to predict short-term all-cause mortality in SAH patient population. Whether this abnormality is a cause of SAH mortality or simply a marker of severe underlying disease remains an unanswered question.
References
Gupte M, John S, Prabhakaran S, Lee VH. Troponin elevation in subarachnoid hemorrhage does not impact in-hospital mortality. Neurocrit Care. 2013;18:368–73.
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–7.
Hunt D, McRae C, Zapf P. Electrocardiographic and serum enzyme changes in subarachnoid hemorrhage. Am Heart J. 1969;77:479–88.
Yoshikawa D, Hara T, Takahashi K, Morita T, Goto F. An association between QTc prolongation and left ventricular hypokinesis during sequential episodes of subarachnoid hemorrhage. Anesth Analg. 1999;89:962.
Zaroff JG, Rordorf GA, Newell JB, Ogilvy CS, Levinson JR. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999;44:34–9.
van der Bilt IA, Hasan D, Vandertop WP, Wilde AA, Algra A, Visser FC, Rinkel GJ. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72:635–42.
Ichinomiya T, Terao Y, Miura K, Higashijima U, Tanise T, Fukusaki M, Sumikawa K. QTc interval and neurological outcomes in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010;13:347–54.
Marafioti V, Rossi A, Carbone V, Pasqualin A, Vassanelli C. Prolonged QTc interval is a powerful predictor of non-cardiac mortality in patients with aneurysmal subarachnoid hemorrhage independently of traditional risk factors. Int J Cardiol. 2013;170:e5–6.
Sandhu R, Aronow WS, Rajdev A, Sukhija R, Amin H, D’aquila K, Sangha A. Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am J Cardiol. 2008;102:632–4.
Ramappa P, Thatai D, Coplin W, Gellman S, Carhuapoma JR, Quah R, Atkinson B, Marsh JD. Cardiac troponin-I: a predictor of prognosis in subarachnoid hemorrhage. Neurocrit Care. 2008;8:398–403.
van der Bilt I, Hasan D, van den Brink R, Cramer MJ, van der Jagt M, van Kooten F, Meertens J, van den Berg M, Groen R, Ten Cate F, Kamp O, Götte M, Horn J, Groeneveld J, Vandertop P, Algra A, Visser F, Wilde A, Rinkel G. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: relationship with outcome. Neurology. 2014;82:351–8.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9.
Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7.
Bazett H. An analysis of the time-relationships of electrocardiograms. Heart. 1920;7:352–70.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184:895–9.
Estanol BV, Marin OS. Cardiac arrhythmias and sudden death in subarachnoid hemorrhage. Stroke. 1975;6:382–6.
Frontera JA, Parra A, Shimbo D, Fernandez A, Schmidt JM, Peter P, Claassen J, Wartenberg KE, Rincon F, Badjatia N, Naidech A, Connolly ES, Mayer SA. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26:71–8.
Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–6.
Elgendy AY, Elgendy IY, Mansoor H, Mahmoud AN. Clinical presentations and outcomes of Takotsubo syndrome in the setting of subarachnoid hemorrhage: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2016. doi:10.1177/2048872616679792.
Okin PM. QT interval prolongation and prognosis: further validation of the quantitative approach to electrocardiography. J Am Coll Cardiol. 2004;43:572–5.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Ahmed N. Mahmoud, Akram Y. Elgendy, Hend Mansoor, and Islam Y. Elgendy have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. The study protocol was approved by the University of Florida Institutional Review Board, which has waived the informed consent given the retrospective nature of the study.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6237F0602739B8E3.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mahmoud, A.N., Elgendy, A.Y., Mansoor, H. et al. Cardiovascular Abnormalities and in-Hospital All-Cause Mortality in Patients with Spontaneous Sub-Arachnoid Hemorrhage: An Observational Study. Cardiol Ther 6, 33–40 (2017). https://doi.org/10.1007/s40119-016-0076-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-016-0076-0