Abstract
Silver nanoparticles (Ag NPs) were synthesized using Alternanthera sessilis leaf and Oregano root extract in an eco-friendly fashion and their significant physicochemical and optochemical properties were ascertained for nano-defined characteristics. The UV–visible spectrum showed a single and distinct absorbance peak at 433 nm (Alternanthera sessilis) and 425 nm (Oregano), typical SPR (surface plasmon resonance) for silver. Structural studies revealed nano-crystal with face centre cubic (FCC) symmetry with monodispersed nature. SEM studies showed spherical-shaped particles and the purity determined from EDX spectrum. The synthesized Ag NPs showed that antibacterial activity was studied. There was a significant inhibitory effect toward clinically important pathogens viz. B. subtilis, S. aureus, P. aeruginosa, and E. coli exposed to Ag NPs at different concentrations.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
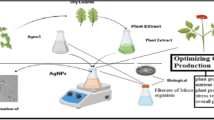
Manoevering of particles at nanoscale tends to gain unique properties for use in various applications, viz., nano-generators, drug delivery system, and medical imaging [1,2,3,4]. Their efficiency to improve solubility, half-life, and sustained release of drugs find its application more appropriate in drug delivery [5]. In the current situation, the nano-biotechnology is one among the most energetic platform, explore the contemporary substantial discipline where the plants and various plant products find an imperious use in the fabrication of nanoparticles [6]. As vast applications of nanomaterials in various fields such as electronic, magnetic, optoelectronics, and information storage are well established. Researchers have found the remarkable application of nanomaterial in the field of medicine such as antimicrobial activity. The existing drug of choice is now becoming a major threat across the globe leading to the emergence of drug-resistant microbes or superbugs challenging the survival of humans. Therefore, to counteract the situation, researchers are on the verge of finding an alternate drug [7]. One such strategy is by the use of metals such as copper, zinc, titanium, magnesium, gold, etc., which, in their nano-form, are considered to exhibit remarkable physical, chemical, and biological properties. To counterbalance the situation, antimicrobial properties of the metallic nanoparticles have been explored to contain resistant strains [8]. Amongst them, silver nanoparticles are found to exhibit unique properties such as conductivity, chemical stability, and catalytic and biological (antibacterial, antiviral, antifungal, and anti-inflammatory) activities [9]. Despite various other methods of synthesizing nanoparticles, the eco-benign approach has been the most sought after. Besides, microbial synthesis, plant product/phytochemical-mediated synthesis of nanoparticles has drawn much attention because of its availability, reproducibility, and reliability [10,11,12,13,14,15,16,17]. This study reports on the eco-friendly green syntheses of silver nanoparticles (Ag NPs) using two plant extracts such as Alternanthera sessilis leaf and Oregano root extracts as a reduction agent. In our study, the role of the extracts in reducing Ag+ to Ag0 has been investigated through spectroscopic and microscopic analyses emphasizing the antibacterial efficacy. The schematic representation of Ag NPs extracted from plants, as shown in Fig. 1.
Materials and methods
Materials
Alternanthera sessilis leaves were procured from a local market in periyakulam, and silver nitrate (AgNO3 ≥ 99.8%; AR grade) was purchased from Sigma-Aldrich and used without further purification. All the glass wares used were cleaned in chromic acid and autoclaved.
Preparation of the extracts of Alternanthera sessilis and Oregano root
50 g of fresh Alternanthera sessilis leaves and Oregano root were thoroughly washed in running water followed by distilled water to remove any dust particles. They were initially dried on an absorbent paper and chopped into small pieces using a pair of scissors. Prior to surface cleansing, Alternanthera sessilis leaves and Oregano roots were blended separately in a mixer grinder for less than a minute with 10 mL of distilled water. The blending was checked for paste-like consistency and collected in two separate Erlenmeyer flasks. To the pastes, 100 mL of double distilled water was added and kept in a shaking incubator at 80 °C for 10 min. Both the mixtures were brought down to room temperature and subjected to filtration using syringe filter (pore size 0.2 µm) and the filtrate maintained at 4 °C.
Synthesis of Ag NPs by Alternanthera sessilis leaf and Oregano root extracts
To 100 mL of 1 mM silver nitrate taken in two separate flasks, 5–10 mL of Alternanthera sessilis leaf and Oregano extracts were added under the stirring condition for 20 min at 60 °C. Reaction pertaining to nanoparticle synthesis with respect to time was observed. Furthermore, separation and purification of the colloids were performed using repeated washing and centrifugation. Finally, the particles were dried and stored in airtight containers for further experiment.
Characterization Techniques
UV–Vis Schimadzu 1800 spectrophotometer was used to record the absorbance spectrum (200–700 nm) of the synthesized silver nanoparticles operated at a resolution of 1 nm. The phase purity of synthesized silver nanoparticles was determined using a Philips X’pert Pro diffractometer (Schimadzu) aided with CuKβ radiation. FT-IR spectrum was recorded on JASCO 4400 in the spectral range of 4000–400 cm−1 with sample pelleted using potassium bromide (1:100). Electron microscopic studies (Carl Zeiss MA15) were performed on the sample sputtered with gold to analyze the surface properties and assembly characteristics. The composition of the synthesized silver nanoparticles was determined using X-ray Energy Dispersion Spectroscopy (Inca, Oxford Instruments, Buckinghamshire, UK).
Antibacterial activity
The antimicrobial activity of phytochemically synthesized silver colloids was determined using agar diffusion method using clinically important pathogens containing Gram-positive and Gram-negative test strains. The pure cultures of the strains at 1 × 108 CFU/mL were swabbed uniformly onto the Mueller–Hinton agar (MHA) medium using sterile swabs. Four hollow blocks of medium (dia 6 mm) were cut from the MHA plates using 100 µL sterile pipette tips. Ag NPs synthesized from two different extracts at a concentration of 25, 50, and 75 µL was added to the wells using a sterile micropipette. Amoxicillin antibiotic (25 µL) was used as a positive control. The culture-inoculated plates treated with Ag NPs and antibiotic were incubated for 18–24 h at 37 °C for the observation of any inhibition zone.
Results and discussion
Structural analysis
The typical XRD pattern of silver nanoparticles synthesized using a leaf and root extracts of Alternanthera sessilis and Oregano root, respectively, is shown in Fig. 2a, b. Both ASL- and OR-mediated synthesis showed diffraction peaks at 2θ = 38.2°, 44°, 64.4°, and 77.2° corresponding to (111), (200), (220), and (311) that can be assigned to face centered cubic symmetry (fcc) [18]. These diffraction patterns were compared and found closely associated to JCPDS No. 04-0783 [19]. The robust intensity of diffraction at 38° indicated silver crystal’s preferential orientation along (111) plane. In addition, we could observe a peak at 2θ = 46.3° which might have been associated with bio-organic phase crystallization [20]. The mean crystallite size of the silver nanoparticles was calculated from Scherrer’s formula [21]:
where D denotes mean crystallite size of the nanoparticle, λ denotes wavelength of X-ray, β accounts for full width at half maximum intensity (FWHM), and θ represent Bragg’s angle. Accordingly, the mean crystal size of the ASL- and OR-mediated nanoparticle synthesis worked out to 19 nm and 10 nm.
Optical studies
A dark brown suspension of colloids was formed after the addition of the extracts to the reaction mixture. This dark brown color may be due to the collective vibrations of the charged particles present on the surface of nanoparticles and the resonance [22,23,24]. A sharp and a narrow distinct peak at λmax = 433 nm in the visible region clearly elucidate the formation of Ag NPs in a short duration (10–15 min) after the addition of ASL extract as in Fig. 3a, b [25]. Similarly, UV–visible spectra recorded an absorbance of 425 nm within 10 min of addition of OR extract as in Fig. 3c, d.
SEM and EDX analysis
The morphology and the elemental composition of Ag NPs were observed using SEM and EDX studies as shown in Figs. 4 and 5. Electron micrograph revealed spherical-shaped particles with a smooth surface and closely arranged [26]. The size of Ag NPs synthesized using ASL and OR extract was around 23.44 nm and 17.58 nm, respectively, which is in good agreement with the crystallite size as inferred by XRD. The elemental composition as demonstrated from EDX spectra revealed a strong signal for silver. In addition, carbon, oxygen, and nitrogen signals could also be detected. Carbon signal might have resulted from the grid, oxides during sample preparation, and nitrogen might have been a phytochemical moiety responsible for capping nanoparticle as shown in inset Figs. 4 and 5.
Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectroscopy exhibited the possible biomolecular interaction in the formation of nanoparticles using ASL and OR (Fig. 6a, d. The FT-IR spectra of OR Ag NPs showed prominent peaks at 3452 cm−1, 2072 cm−1, 1634 cm−1, and 642 cm−1 attributing N–H asymmetric stretching assigned to Amide group, C=C-stretching vibration denoting Alkyne group, N–H bend indicating primary amine group, and C–Br stretching vibration indicating the Alkyl halide group. A peak residing at 655 cm−1 represented a key component responsible for the reduction and capping of the extract with that of metal by their intermolecular interaction [27]. The sharp absorption peak at 664 cm−1 can be attributed due to C–Cl stretching for halogen compounds, the alcohol and phenols stretching of C–O bond occur at 1074 cm−1, the band at 1615 cm−1 exist due to C=O stretch of tertiary amides, and the broad peak at 3426 cm−1 is the characteristic O–H stretching for alcohols and phenols. In addition, the functional biomolecules in ASL extract were hydroxyl, carboxylic, phenol, and amine groups involved in silver ion reduction. Thus, biological molecules enforce the dual role of formation and stabilization of Ag NPs in the aqueous medium [28].
Antimicrobial assay
The antimicrobial activity of ASL- and OR-mediated Ag NPs is elaborated in Figs. 7 and 8. There was a significant inhibition of growth of bacteria tested. ASL-mediated Ag NPs showed the maximum inhibition zone against Staphylococcus aureus (11 mm) followed by Pseudomonas aeruginosa (10 mm), Escherichia coli (9 mm), and Bacillus subtilis (4 mm). OR-mediated Ag NPs susceptibility pattern showed a maximum zone toward Pseudomonas aeruginosa (6 mm) followed by Escherichia coli (5 mm), Bacillus subtilis (4 mm), and Staphylococcus aureus (3 mm).
Antibacterial mechanism exhibited by metal nanoparticles depends on the degree of susceptibility of microbes. The nanoparticles when encountered with the microbe adhere to the bacterial surface via electrostatic interaction. Their significantly smaller size helps gain entry into the bacterial cell via the transmembrane proteins and by the influence of proton motive force. It has a greater affinity towards sulfur groups present in proteins to form thiols [29] and on phosphates forming complexes resulting in DNA damage. It was demonstrated that Ag NPs’ interaction with cysteine residues results in the generation of ROS by inhibiting electrons at terminal oxidase, thereby inducing bacterial cell death. The difference in susceptibility pattern toward Ag NPs exposed to Gram-positive and Gram-negative strains relies on their cell wall make up. Gram-positive strains possess a thick cell wall made of peptidoglycan that helps to prevent intrusion of foreign body selectively, whereas the Gram-negative strain lacks such component that falls easy prey to an antimicrobial agent, in this case, Ag NPs, which sustains severe damage leading to cell death [30,31,32,33].
Conclusions
The present work highlights the most simple and economical approach in the synthesis of Ag NPs using plant extracts of Alternanthera sessilis (leaf) and Oregano (root) as reducing agents. Spectroscopic and Microscopic analyses revealed typical nano-characteristics of silver. FT-IR results confirmed the contribution of phytochemicals, viz., terpenes, flavonoids, and proteins for effective synthesis. There was a significant bactericidal activity against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli as evidenced from agar well-diffusion method. This biosynthesized Ag NPs represented a promising antimicrobial with potential biomedical applications. Therefore, this green chemistry approach towards the synthesis of Ag NPs has been the most sought-after method in terms of economic viability.
References
De-Zhi, L., Hong-Da, C., Feng, B., Zhen-Xin, W.: Progress of multimodal molecular imaging technology in diagnosis of tumor. Chin. J Anal. Chem. 44, 1609–1618 (2016). https://doi.org/10.1016/S1872-2040(16)60966-0
Ujjal Kumar, S., Balaprasad, A., Sanat, K., Animesh, H., Pulak, D.: Green synthesis of silver nanoparticles using the plant extract of Shikakai and Reetha. Mater. Today Proc. 5, 2321–2329 (2018). https://doi.org/10.1016/j.matpr.2017.09.236
Thombre, R., Mehta, S., Mohite, J., Jaisinghani, P.: Synthesis of silver nanoparticles and its cytotoxic effect against thp-1 cancer cell line. Int. J. Pharma Bio Sci. 4(1), 184–192 (2013)
Naraginti, S., Li, Y.: Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B 170, 225–234 (2017). https://doi.org/10.1016/j.jphotobiol.2017.03.023
Dakshayani, S.S., Marulasiddeshwara, M.B., Ramesh, M.N.S., Raghavendra Kumar, P., Devaraja, S.R.H.K.: Antimicrobial, anticoagulant and antiplatelet activities of green synthesized silver nanoparticles using Selaginella (Sanjeevini) plant extract. Int. J. Biol. Macromol. 131, 787–797 (2019). https://doi.org/10.1016/j.ijbiomac.2019.01.222
Zahra, H.P., Hossein, A., Naser, K., Ali, F.: Eco-Friendly synthesis and antimicrobial activity of silver nanoparticles using Dracocephalum moldavica seed extract. Appl. Sci. 6, 69 (2016). https://doi.org/10.3390/app6030069
Marulasiddeshwara, M.B., Dakshayani, S.S., Sharath Kumar, M.N., Chethana, R., Raghavendra Kumar, P., Devaraja, S.: Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: a promising therapeutic agent. Mater. Sci. Eng. C Mater. Biol. Appl. 81, 182–190 (2017). https://doi.org/10.1016/j.msec.2017.07.054
Khalil, K.A., Fouad, H., Elsarnagawy, T., Almajhdi, F.N.: Preparation and characterization of electrospun PLGA/silver composite nanofibers for biomedical applications. Int. J. Electrochem. Sci. 8, 3483–3493 (2013)
Kamiar, Z., Seyedmohammad, P., Arman, S., Pouyan, M., Keyvan, P., Mohammad, J.R., Ali, A.M.: Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed. Res. Int. (2016). https://doi.org/10.1155/2016/5435397
Nakkala, J.R., Mata, R., Gupta, A.K., Sadras, S.R.: Biological activities of green silver nanoparticles synthesized with Acorus calamus rhizome extract. Eur. J. Med. Chem. 85, 784–794 (2014). https://doi.org/10.1016/j.ejmech.2014.08.024
Nabikhan, A., Kandasamy, K., Raj, A., Alikunhi, N.M.: Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B Biointerfaces. 79(2), 488–493 (2010). https://doi.org/10.1016/j.colsurfb.2010.05.018
Ulug, B., Haluk, Turkdemir, Cicek, A., Mete, A.: Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 25(135), 153–161 (2015). https://doi.org/10.1016/j.saa.2014.06.142
Hossam, E.E., Manal, M.E., Hanan, B.A.: One-pot fabrication of AgNPs, AuNPs and Ag-Au nano-alloy using cellulosic solid support for catalytic reduction application. Carbohydr. Polym. 166, 1–13 (2017). https://doi.org/10.1016/j.carbpol.2017.02.091
Yosari, S., Pontaza-Licona Ramos-Jacques, A.L., Cervantes-Chavez, J.A., Luis López-Miranda, J., Álvaro de Jesús, R.B., Maya-Cornejo, J., Rodríguez-Morales, A.L., Esparza, R., Estevez, M., Pérez, R., Hernandez-Martínez, A.R.: Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Results Phys. 12, 1670–1679 (2019). https://doi.org/10.1016/j.rinp.2019.01.082
Rocha-Rocha, O., Cortez-Valadez, M., Hernandez-Martinez, A.R., Gamez-Corrales, R., Alvarez, R.A.B., Britto-Hurtado, R., Delgado-Beleño, Y., Martinez-Nuñez, C.E., Pérez-Rodríguez, A., Arizpe-Chávez, H., Flores-Acosta, M.: Green synthesis of Ag–Cu nanoalloys using Opuntia ficus-indica. J. Electron. Mater. 46, 802 (2017). https://doi.org/10.1007/s11664-016-4942-2
Haydé, V.C., Granados-Segura, L.O., Gabriel, L.B., David, J.M., María, G.H., Noe, A., Angel, R., Miriam, E., Héctor, P.: Gold nanoparticles bioreduced by natural extracts of arantho (Kalanchoe daigremontiana) for biological purposes: physicochemical, antioxidant and antiproliferative evaluations. Mater. Res. Express 6, 055010 (2019). https://doi.org/10.1088/2053-1591/ab0155
Geetha, R., Thirunavukkarasu, A., Tamilselvan, S., Govindaraju, K., Sadiq, M., Singaravelu, G.: Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. (2013). https://doi.org/10.1007/s12645-013-0040-9
Roy, K., Sarkar, C.K., Ghosh, C.K.: Plant-mediated synthesis of silver nanoparticles using parsley (Petroselinum crispum) leaf extract: spectral analysis of the particles and antibacterial study. Appl. Nanosci. 5, 945–951 (2015). https://doi.org/10.1007/s13204-014-0393-3
Elumalai, D., Hemavathi, M., Deepaa, C.V., Kaleena, P.K.: Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control. 2(4), 15–26 (2017). https://doi.org/10.1016/j.parepi.2017.09.001
Mallikarjuna, K., Sushma, N.J., Narasimha, G., Manoj, L., Raju, B.D.P.: Phytochemical fabrication and characterization of silver nanoparticles by using Pepper leaf broth. Arab. J. Chem. 7, 1099–1103 (2014). https://doi.org/10.1016/j.arabjc.2012.04.001
Rajkumar, P.V., Ravichandran, K., Baneto, M., Ravidhas, C., Sakthivel, B., Dineshbabu, N.: Enhancement of optical and electrical properties of SILAR deposited ZnO thin films through fluorine doping and vacuum annealing for photovoltaic applications. Mater. Sci. Semicond. Process. 35, 189–196 (2015). https://doi.org/10.1016/j.mssp.2015.03.010
Ahamed, M., Khan, M.A.M., Siddiqui, M.K.J., AlSalhi, M.S., Alrokayan, S.A.: Green synthesis, characterization, and evaluation of biocompatibility of silver nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 43(6), 1266–1271 (2011). https://doi.org/10.1016/j.physe.2011.02.014
Krishnaraj, C., Jagan, E.G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P.T., Mohan, N.: Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 76(1), 50–56 (2010). https://doi.org/10.1016/j.colsurfb.2009.10.008
de Matos, R.A., da Silva Cordeiro, T., Samad, R.E., Vieira, N.D.: Green synthesis of stable silver nanoparticles using Euphorbia milii latex. Colloids Surf. A 389(1–3), 134–137 (2011). https://doi.org/10.1016/j.colsurfa.2011.08.040
Vidhu, V.K., Aromal, S.A., Philip, D.: Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 83(1), 392–397 (2011). https://doi.org/10.1016/j.saa.2011.08.051
Banerjee, P., Satapathy, M., Mukhopahayay, A., Das, P.: Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 1, 1–10 (2014). https://doi.org/10.1186/s40643-014-0003-y
Iravani, S.: Green synthesis of metal nanoparticles using plants. Green Chem. 13, 2638–2650 (2011). https://doi.org/10.1039/c1gc15386b
Ajitha, B., Reddy, A.K., Reddy, P.S.: Biosynthesis of silver nanoparticles using Momordica charantia leaf broth: evaluation of their innate antimicrobial and catalytic activities. J. Photochem. Photobiol. B Biol. 146, 1–9 (2015). https://doi.org/10.1016/j.jphotobiol.2015.02.017
Patil, R.S., Kokate, M.R., Kolekar, S.S.: Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 91, 234–238 (2012). https://doi.org/10.1016/j.saa.2012.02.009
Snega, S., Ravichandran, K., Baneto, M., Vijayakumar, S.: Simultaneous enhancement of transparent and antibacterial properties of ZnO films by suitable F doping. J. Mater. Sci. Technol. 31, 759–765 (2015). https://doi.org/10.1016/j.jmst.2015.03.001
Ravichandran, K., Snega, S., Jabena Begum, N., Swaminathan, K., Sakthivel, B., Rene Christena, L.: Enhancement in the antibacterial efficiency of ZnO nanopowders by tuning the shape of the nanograins through fluorine doping. Superlattice Microstruct. 69, 17–28 (2014). https://doi.org/10.1016/j.spmi.2014.01.020
Nikparast, Y., Saliani, M.: synergistic effect between phyto-synthesized silver nanoparticles and ciprofloxacin antibiotic on some pathogenic bacterial strains. J. Med. Bacteriol. 7, 36–43 (2018)
Sondi, I., Salopek-Sondi, B.: Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275(1), 177–182 (2004). https://doi.org/10.1016/j.jcis.2004.02.012
Acknowledgements
One of the authors (Suresh Sagadevan) acknowledges the honor, namely the “Senior Research Fellow” at Nanotechnology & Catalysis Research Centre (NANOCAT), University of Malaya 50603 Kuala Lumpur, Malaysia. The author wishes to place on record his heartfelt thanks that are due to the authorities concerned.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Selvaraj, V., Sagadevan, S., Muthukrishnan, L. et al. Eco-friendly approach in synthesis of silver nanoparticles and evaluation of optical, surface morphological and antimicrobial properties. J Nanostruct Chem 9, 153–162 (2019). https://doi.org/10.1007/s40097-019-0306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-019-0306-9