Abstract
As the nano revolution unfolds, it is imperative to integrate nanoscience and medicine. The secret gleaned from nature have led to the generation of biogenic technologies for the fabrication of advanced nanomaterials. Present investigation discloses the gold nanoparticles biosynthesizing capability of the flower of pharmacologically important tree Couroupita guianensis. Rapid, cost-effective, one-step process of synthesis has been achieved. Newly genre gold nanoparticles were characterized by involving UV–vis spectroscopy, FTIR, XRD, SEM, and TEM analysis. Interestingly, as a result of extensive screening on the application of newly synthesized gold nanoparticles their anticancer potential has been discovered using MTT assay, DNA fragmentation, apoptosis by DAPI staining, and comet assay for DNA damage.
Similar content being viewed by others
1 Introduction
Metal nanoparticles are of great importance due to their high surface area and a high fraction of surface atoms. The scientific and technological significance of metal nanoparticles has made them the subject of intensive research, given their special chemical and physical properties. In particular, gold nanoparticles are employed in many fields: biosensing (Mirkin et al. 1996), catalysis (Sen et al. 2012), electronics (Rao and Cheetham 2001), enzyme electrodes (Crumbliss et al. 1992), super conductors (Sun and Xia 2002), and cancer therapy (EI-Sayed et al. 2006) among others.
Presently, biological nanoscience has drawn increasing attention due to its avant-garde nature and the efficacy of produced nanoparticles in industrial, biomedical, and electronic applications such as catalyst (Hashmi and Hutchings 2006), cancer detection (Huang et al. 2007b), and bioimaging (Wu et al. 2006). Among the loads of metal nanoparticles, gold nanoparticles have an especially wide application in DNA recognition, heredity medicine, and nano-catalysis. It has been pointed out that the divergent capabilities of these nanoparticles are controlled by their size (Alivisatos 1996; Jin et al. 2001; Aizpurua et al. 2003).
Numerous methodologies are developed to synthesize noble metal nanoparticles of particular shape and size depending on specific requirements. Biosynthesis of nanoparticles has an emerging highlight of the intersection of nanotechnology and biotechnology which has received increased attention to a growing need to develop environmentally benign technologies in material syntheses. Biomolecules as reductants are found to have significant advantage over chemical reductants due to their non biocompatible nature (Huang et al. 2007a).
In the present cram, a simple biosynthetic method using flower extract of Couroupita guianensis is addressed herein for synthesizing gold nanoparticles. The newly synthesized gold nanoparticles were characterized by employing standard technologies. The plant C. guianensis, commonly known as cannon ball tree, has innumerable medicinal applications including antibiotic, antiseptic, anti-inflammatory activity (Geetha et al. 2004). Indeed, it is expected that gold nanoparticles synthesized using the flower of the tree C. guianensis might have medicinal properties. Accordingly, efforts are made to identify the newly generated gold nanoparticles’ medicinal value. As a result, their antileukemic cancer activity has been explored. Cancer is the leading cause of death worldwide after cardiovascular diseases (Garrett and Workman 1999). Leukemia is a major type of cancer affecting a significant segment of the population, especially children. In fact, leukemia is the most frequent childhood cancer, with 26 % of all cases, and 30 % mortality (Canadian Cancer Society 2005). Clinical trials in identifying new agents and treatment modalities has been studied and achieved. However, current treatments have many limitations related to their side effects and the development of acquired drug resistance (Robert and Jarry 2003). Thus, the new therapeutic agents needed should be more active and produce fewer side effects and they also should act through a mechanism different from that of cytotoxic agents already used.
2 Materials and methods
2.1 Preparation of flower extract
Fresh flowers of C. guianensis were collected, washed thoroughly with double distilled water, air dried, and powdered. About 1 g of powdered flower was transferred into a 100-mL beaker containing 50 mL double distilled water and mixed well. The extract obtained was filtered through Whatmann no. 1 filter paper and the filtrate was collected in a separate flask.
2.2 Synthesis of gold nanoparticles using plant extract
One hundred milliliter solution of 1 mM chloroauric acid at concentration of 10−3 M was prepared by dissolving in double distilled water. Different reaction concentrations of C. guianensis extract and HAuCl4 solution (1:9, 2:8, 3:7, 4:6, and 5:5) were subjected, respectively. The reduction of gold ions to gold nanoparticles was completed within 5 min. The pale yellow color solution turns to ruby red color indicating the formation of gold nanoparticles. The reduction of metal ions was continuously monitored by visual inspection as well as by measuring with UV–visible spectrometer in the wavelength range 300–800 nm.
2.3 Characterization of gold nanoparticles
UV–visible spectrum was recorded as a function of the reaction time on a Techomp (UV 2300) double beam spectrophotometer.
For FTIR measurements, the newly synthesized gold nanoparticles were centrifuged at 8,000 rpm for 10 min and the resulting suspension was redispersed in distilled water. The purified pellets were dried and smirk with KBr pellets and analyzed by Thermo Nicolet Quator instrument in the diffuse reflectance mode operating at a resolution of 4 cm−1.
XRD measurements of the C. guianensis-synthesized gold nanoparticles solution drop coated on to glass substrates was done on a Siefert X-ray diffractometer, operated at a voltage of 40 kV and tube current of 30 mA with Cu-Kα radiation. Surface morphology of newly formed gold nanoparticles was determined using scanning electron microscope (FEI Quanta 200 SEM).
For TEM studies, newly synthesized gold nanoparticles were prepared by placing a drop of nanoparticle solution on carbon-coated copper grids and allowing water to evaporate. TEM measurements were performed on JEOL 3010 UHR TEM equipped with a Gatan Imaging Filter.
2.3.1 Assessment of cell morphology
HL-60 cells were cultured in 24-well plates and incubated at 37 °C for 24 h before each well were individually treated with 0, 60, 90, 120, 150, and 180 μg/mL gold nanoparticles for 48 h. Determination of cell morphology was made using a phase contrast microscope (Lu et al. 2010).
2.3.2 DAPI staining for apoptosis
Cells in 12-well plates were treated with or without 0, 80, 120, 180, and 200 μg/mL gold nanoparticles for 48 h. Cells were then stained by using 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Chen et al. 2009a).
2.3.3 Comet assay for DNA damage
Cells in 96-well plates were treated with or without 0, 60, 90, 120, 150, and 180 μg/mL gold nanoparticles for 48 h. Cells were then harvested and DNA damage was determined with the Comet assay (Chen et al. 2009b).
2.3.4 DNA gel electrophoresis for DNA fragmentation
Cells in six-well plates were incubated with 0 and 150 μg/mL gold nanoparticles 48 h. Cells were harvested by centrifugation and DNA was isolated before DNA fragmentation was determined by DNA gel electrophoresis (Lu et al. 2010).
2.3.5 Effect of gold nanoparticles on cytotoxicity
HL-60 cells were obtained from the NCCS, Pune, India, and grown in RPMI1640 medium and supplemented with a 2 mg mL−1 sodium bicarbonate, 4.5 mg mL−1 glucose, 100 μg mL−1 streptomycin sulfate, 40 μg mL−1 gentamycin, 100 U mL−1 penicillin as well as 10 % heat-inactivated fetal calf serum. An environment of humidified air containing 5 % CO2 was maintained at 37 °C. Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and cultured as described. Effects of gold nanoparticles on the proliferation of cells was determined by MTT [3-(4, 5-dimethyl thiazoyl-2-yl)-(2,5-diphenyltetrazolium bromide)] assay. Briefly, the cells were suspended at 3 × 105 Cell mL−1. The cells were placed in 96-well microtiter plates (200 μl well−1) and incubated at 37 °C in a CO2 incubator in the presence of the gold nanoparticles. After 5 days, cell viability was measured by MTT assay, from which cytotoxic concentration (CC50) was calculated (Premnathan et al. 1996).
3 Results and discussion
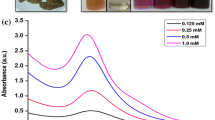
Design and development of nanomaterials with unusual physico-chemical and optoelectronic features is a corner stone in nanoscience. Present report discloses the bioreduction property of gold ions into gold nanoparticles by ethnopharmacologically important C. guianensis flower’s extract. Synthesis of gold nanoparticles has been achieved in 5 min of reaction without any experimental protocols like stirring, heating, change in pH. Reduction and capping of newly formed gold nanoparticles was achieved with the flower extract of C. guianensis. As a result of extensive screening with different concentrations of auric chloride and flower extract, synthesis was successful with the concentration of 2:8 (2 mL of flower extract and 8 mL of auric chloride). Figure 1 shows the UV–vis spectra of the newly formed gold nanoparticles and the characteristic SPR band occurs at ca. 534 and at 5 min that attained the maximum intensity.
Fourier transform infrared (FTIR) was made in order to identify the possible biomolecules responsible for the reduction of gold ions and capping of the bioreduced gold nanoparticles (Fig. 2 a and b). FTIR spectrum of the aqueous extract of C. guianensis (curve a) showed peaks at 3,414 (O–H stretch, H–bonded of alcohols/phenols), 2,133 (−C (triple bond) C– stretch of alkynes), 1,648 (−C=C– stretch of alkynes), 1,383 (C–N stretching vibration of aliphatic amines), 1,288 (C–O stretch of alcohols), and 1,073 cm−1 (C–N stretching vibration of aliphatic amines or alcohols/phenols). The intense broad absorbance at 3,414 cm−1 is characteristic of the hydroxyl functional group in alcohols and phenolic compounds. The FTIR spectrum of the gold nanoparticles (curve b) showed peaks at 3,615 and 3,547 (O–H stretch, H–bonded of alcohols/phenols), 2,924 and 2,855 (C–H stretch), 2,402 and 2,129 (−C (triple bond) C– stretch of alkynes), 1,744 (C=O stretch of carboxylic acids), 1,378 (C–N stretching vibration of aliphatic amines), and 1,071 cm−1 (C–N stretching vibration of aliphatic amines or to alcohols/phenols). Comparing both FTIR spectra it can be identified that the changes in the –COOH group for –OH, i.e., hydroxyl group the peak appeared at 3,414 cm−1 in raw material, but after encapsulation of nanoparticles, the peak is narrower and shifted to 3,615 cm−1 and also for –C– of carboxylic group the peak intensity reduced after encapsulation of nanoparticles. The peaks at 1,648, 1,383, 1,288, and 1,073 cm−1 are reduced and the new peak appeared at 1,744 cm−1 which indicates the alcoholic group is converted into aldehyde to reduce Au3+ to Au0.
A structural analysis of gold nanoparticles prepared from the sample was performed by XRD. Taking into account the angular positions of the Bragg peaks (Fig. 3), a face-centered cubic structure was assigned to the gold nanoparticles. The XRD pattern thus clearly illustrates that the gold nanoparticles synthesized by C. guianensis are crystalline in nature.
Figure 4 shows the SEM images of the newly synthesized gold nanoparticles. It was observed that the surface morphology of gold nanoparticles synthesized from flower extract of C. guianensis solution clearly indicates that they are in different shapes and well dispersed.
The TEM images confirm the formation of gold nanoparticles and it has been observed that the newly formed nanoparticles are polydispersed consisting of spherical, triangular, tetragonal and pentagonal with irregular contours. The size distribution of gold nanoparticles diameter is located between 7 and 48 nm (Fig. 5). Generation of metal nanoparticles mainly relies on the use of lethal reducing agents. For the development of biocompatible nanoparticles, various biomimetic approaches are being explored. Indeed, a discovery on the application of nanoparticles in medical sciences is exciting which is continually strengthened by the ever-widening spectrum. This necessitates the need of biologically benign nanoparticles. Biological methods may provide an alternate to the conventional techniques, with emphasis on the production of environmentally benign nanoparticles. However, the limitations such as change in pH and incubation are accountable in the large-scale production. Greener technology on the synthesis of nanoparticles deserves merit to overcome the drawbacks of microbial synthesis of nanoparticles. The result addressed herein is on the biological synthesis of gold nanoparticles using medicinally important tree C. guianensis flower extract.
There are reports on the plant-mediated synthesis of gold nanoparticles. Ghoreshi and co-workers (2011) reported on the synthesis of gold nanoparticles using the flower extract of Rosa damascene. Studies also demonstrate that the flavanoids and polyphenols of the flower are responsible for the formation of quasi-spherical nanoparticles. Philip (2010) demonstrated Hibiscus rosa sinensis-mediated synthesis of gold nanoparticles and it has been reported that carboxylic group of malic acid may be the active principle for the reduction and capping of gold nanoparticles. Cinnamomum zeylanicum-mediated synthesis of gold nanoparticles have been reported and possibly the amines of proteins bound the newly formed nanoparticles (Smitha et al. 2009). It has been illustrated that flavanoids of the plant Syzygium aromaticum found to have the property of synthesizing irregular-shaped nanoparticles (Deshpande et al. 2010). Pioneering works on nanoparticles synthesis using plant extracts have been carried out by Sastry and co-workers using geranium leaves, neem, Aloe vera, and lemon grass are extensively studied (Ankamwar et al. 2005; Shankar et al. 2004; Chandran et al. 2006; Shankar et al. 2005). Singaravelu and co-workers (Singaravelu et al. 2007, Govindaraju et al. 2008, Khaleel et al. 2010) reported on the biological synthesis of gold and silver nanoparticles using Sargassum wightii, Spirulina platensis, Psidium guajava as green chemistry approach.
Foregoing facts discloses that phytochemicals in the synthesis of nanoparticles is an important symbiosis between nanoscience and green chemistry. As a result of our extensive screening on identifying the newly formed gold nanoparticles application, anti leukemic cancer activity has been explored. HL-60 cells were treated with various concentrations of gold nanoparticles for different time-periods. The results shown in Fig. 6a and b indicate the nuclear morphology of dying HL-60 cells due to gold nanoparticles treatment and these effects were dose-dependent. Figure 7a and b indicates that gold nanoparticles induced apoptosis in HL-60 cells, and the results showed less cell number compared to control. Gold nanoparticles induced apoptosis which was consistent with data showing that DNA fragmentation occurred as seen in Fig. 8a and b. Concentrations of gold nanoparticles led to a longer tail (DNA damage), further supporting the fact that gold nanoparticles induced apoptosis in HL-60 cells and data in Fig. 9 show DNA gel electrophoresis of DNA fragmentation which was enhanced with increasing exposure to gold nanoparticles.
Cell viability determination was made using MTT assay which was conducted for 120 h. C. guianensis flower extract-synthesized gold nanoparticles caused a significant cytotoxicity in HL-60 cells in a concentration-dependent manner with the CC50 value of 5.14 μM whereas for PBMC it is 113.25 μM (Fig. 10).
Despite the widespread use of gold nanoparticles, there are only few studies to determine the cytotoxic effects of biologically synthesized gold nanoparticles, particularly in the context of apoptosis. MTT assay was used to appraise the effect of gold nanoparticles on proliferation of HL-60 cells. This is the first study to evaluate C. guianensis-based gold nanoparticles’ cytotoxicity against cancer cell lines. Thus we hypothesize that cell killing could be the possible mechanism induced by the cytotoxic effect of biosynthesized gold nanoparticles.
Nanotechnology has attracted a great interest in recent years due to its expected impact on many areas such as energy, medicine, and electronics. The development of new materials with nanometer size including nanoparticles, nanotubes, nanowires, etc., is the major activity. Among all, nanoparticles with the unique properties in chemistry, optics, electronics, and magnetics have led to an increasing interest in their synthesis.
Much of the existing research in the development of anticancer therapeutics from the plants has been focused on investigating the molecular mechanism by which an agent induces cytotoxicity and apoptosis in cancer cells. This study provides valuable insight in developing nanotherapeutics against dreadful cancer.
Investigation suggests that ethnopharmacological approach on the production of gold nanoparticles will greatly facilitate on the creation of an important symbiosis between nanoscience and medical science.
4 Conclusions
The results addressed herein disclose anticancer activity of gold nanoparticles synthesized using aqueous flower extract of C. guianensis. It is reasonable to infer that the pharmacological properties of C. guianensis provide the anticancer activity of newly formed gold nanoparticles. Interestingly, it has been demonstrated that the functionalization of gold nanoparticles as anticancer nanomaterial has been achieved without doping of molecules.
References
Aizpurua J, Hanarp P, Sutherland DS, Kall M, Bryant GW, Garcia de Abajo FJ (2003) Optical properties of gold nanorings. Phys Rev Lett 90:057401-1–057401-4
Alivisatos AP (1996) Perspectives on the physical chemistry of semiconductor nanocrystals. J Phys Chem 100:13226–13239
Ankamwar B, Chaudhary M, Sastry M (2005) Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorg Met Org Nano Metal Chem 35:19–26
Canadian Cancer Society–National Cancer Institute of Canada (2005) Canadian Cancer Statistics 2005, Toronto, Canada, April, ISSN: 0835–2976
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577–583
Chen JC, Lu KW, Lee JH, Yeh CC, Chung JG (2009a) Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res 26:4313–4326
Chen JC, Lu KW, Tsai ML, Hsu SC, Kuo CL, Yang JS, Hsia TC, Yu CS, Chou ST, Kao MC, Chung JG, Wood WG (2009b) Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells. Oral Oncol 45:273–283
Crumbliss AL, Perine SC, Stonehuerner J, Tubergen KR, Zhao J, Henkens RW, O’Daly JP (1992) Colloidal gold as biocompatible immobilization matrix suitable for the fabrication of enzyme electrodes by electrodeposition. Biotechnol Bioeng 40:483–490
Deshpande R, Bedre MD, Basavaraja S, Sawle B, Manjunath SY, Venkataraman A (2010) Rapid biosynthesis of irregular shaped gold nanoparticles from maccrated aqueous extracellular dried clove buds (Sygyium aromaticum) solution. Colloids Surf B: Biointerfaces 79:235–240
EI-Sayed IH, Huang X, EI-Sayed MA (2006) Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett 239(1):129–135
Garrett MD, Workman P (1999) Discovering novel chemotherapeutic drugs for the third millennium. Eur J Cancer 35(14):2010–2030
Geetha M, Saluja AK, Shankar MB, Mehta RS (2004) Analgesic and anti inflammatory activity of Couroupita guianensis Aubl. J Natural Remedies 4(1):52–55
Ghoreishi SM, Behpour M, Khayatkashni M (2011) Green synthesis of silver and gold nanoparticles using Rosa damascene and its primary application in electrochemistry. Physica E 44:97–104
Govindaraju K, Khaleel Basha S, Ganesh Kumar VG, Singaravelu G (2008) Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci 43:5115–5123
Hashmi ASK, Hutchings GJ (2006) Gold catalysis. Angew Chem Int Ed 45:7896–7936
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Shao W, He N, Hong J, Chen C (2007a) Biosynthesis of silver and gold nanoparticles by using novel sun-dried Cinnamomum camphora leaves. Nanotechnol 18:105104–105114
Huang XH, Jain PK, EI-Sayed IH, EI-Sayed MA (2007b) Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine 2:681–693
Jin RC, Cao YW, Mirkin CA, Kelly KL, Schatz GC, Zheng JG (2001) Photoinduced conversion of silver nanospheres to nanoprisms. Science 294:1901–1903
Khaleel Basha S, Govindaraju K, Manikandan R, Ahn J, Bae EY, Singaravelu G (2010) Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf B: Biointerfaces 75:405–409
Lu HF, Lai TY, Hsia TC, Tang YJ, Yang JS, Chiang JH, Lu CC, Liu CM, Wang HL, Chung JG (2010) Danthron induces DNA damage and inhibits DNA repair gene expressions in GBM 8401 human brain glioblastoma multiforms cells. Neurochem Res 35:1105–1110
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJA (1996) DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Philip D (2010) Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E 42:1417–1424
Premnathan M, Nakashima H, Kathiresan K, Rajendran N, Yamamoto N (1996) In vitro anti human immunodeficiency virus activity of mangrove plants. Ind J Med Res 103:278–281
Rao CNR, Cheetham AK (2001) Science and technology of nanomaterials: current state and future prospects. J Mater Chem. 11:2887–2894
Robert J, Jarry C (2003) Multidrug resistance reversal agents. J Med Chem 46(23):4805–4817
Sen IK, Maity K, Islam SS (2012) Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydrate Polymer. doi:10.1016/j.carbpol.08.058
Shankar SS, Rai A, Ahmed A, Sastry M (2005) Controlling the optical properties of lemon grass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem Mater 17:566–572
Shankar S, Rai A, Ahmed A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interf Sci 275:496–502
Singaravelu G, Arockiyamari J, Ganesh Kumar V, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga Sargassum wightii Greville. Colloids Surfaces B: Biointerf 57:97–101
Smitha SL, Philip D, Gopchandran KG (2009) Green synthesis of gold nanoparticles using Cinnamom zeylanicum leaf broth. Spectrochim Acta, Part A Mol Biomol Spec 74:735–739
Sun Y, Xia Y (2002) shape-controlled synthesis of gold and silver nanoparticles. Science 298:2176–2179
Wu YL, Li YN, Liu P, Gardner S, Ong BS (2006) Studies of gold nanoparticles as precursors to printed conductive features for thin-film transistors. Chem Mater 18:4627–4632
Acknowledgment
G.S and R.G thank the INSPIRE—Department of Science and Technology, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Geetha, R., Ashokkumar, T., Tamilselvan, S. et al. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nano 4, 91–98 (2013). https://doi.org/10.1007/s12645-013-0040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12645-013-0040-9