Abstract
A novel nanophotochromic film consisting of Au nanoparticles and Wells–Dawson-type heteropolyacid, [H6P2W18O62], was prepared by combination of sol–gel and photoreduction method. This film was characterized by UV–visible spectroscopy, particle size distribution, Field emission scanning electron microscopy, and energy dispersive spectroscopy analysis. For the preparation of Au nanoparticles, heteropolyacid, [H6P2W18O62] was used in form of composite film as a green reductant and stabilizer. Au nanoparticles with particle size in the range of 10–20 nm were synthesized and monodispersed in the nanocomposite using dip coating method. The photocatalytic activity of this composite film was studied in the decolorization of methyl orange (MeO) and methyl red (MR) as carcinogenic pollutant dyes using UV irradiation. The pseudo-first order rate constants was established and calculated for these reactions. Comparison of this composite film with the prepared composite by Preyssler heteropolyacid disclosed that the structure of heteropolyacid can affect loading amount of Au nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Au nanoparticles have attracted much attention nowadays mainly due to their exceptional physical and chemical properties. Thus, much effort has been made into their synthesis and characterization [1]. Polyoxometalates (POMs) have been extensively used in the preparation of Au nanoparticles reported in various reports [2–5]. The chemistry of POMs and their salts has been extensively reviewed [6, 7]. They have been defined as a sub-class of inorganic metal oxide clusters, which possess fascinating structures and various properties [8]. Interestingly, when they are submitted to stepwise and multi-electron redox reactions, their structures remain intact. In addition, in their presence it is possible to reduce a compound, electrochemically or photochemically using a suitable reducing agent [9, 10]. Mandal et al. [2] performed a simple process and successfully observed that several metal nanoparticles were formed in the presences of photochemically reduced Keggin heteropolyanions as photocatalysts. Mandal and co-workers demonstrated that the reduction of Ag+ on the surface of gold nanoparticles can be facilitated in the presence of photochemically generated [PW12O40]4− under UV irradiation. In addition, Au nanoparticles were readily prepared via a simple photoreduction mediated by transition metal monosubstituted Keggin heteropolyanions [5]. Although Keggin and its derivatives have been used in the synthesis of Au nanoparticles, the role of Wells–Dawson heteropolyacids (HPAs) has been largely disregarded. A heteropolyacid is a class of acid made up of a particular combination of hydrogen and oxygen with certain metals and non-metals. This type of acid is a common re-usable acid catalyst in chemical reactions [5]. Wells–Dawson-type heteropolyacid [H6P2W18O62] is remarkable due to its high acidity and outstanding stability both in solution and in the solid state [11]. Literature survey revealed that in spite of these interesting properties the synthesis of nanoparticles using this heteropolyacid in nanocomposite films has been unstudied. We are interested in the chemistry of HPAs [12]. Armed with these experiences and due to the recent interest in the synthesis of nanoparticles in solid matrices [13], we were encouraged to study the preparation of Au nanoparticles incorporated in Wells–Dawson heteropolyacid nanocomposite film. Recently, we have introduced the Preyssler-type heteropolyacid (H14[NaP5W30O110]), as an unique reducing agent and stabilizer for the synthesis of Au nanoparticles in an organic–inorganic nanocomposite film under UV irradiation [13]. To extend our research to other HPAs, we investigated the capability of Wells–Dawson heteropolyacid in the preparation of Au nanoparticles in an organic–inorganic nanocomposite film.
Experimental
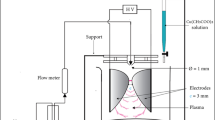
Chemicals and instruments
For the synthesis of Dawson acid, at first potassium salt was synthesized and then Wells–Dawson acid, [H6P2W18O62], was prepared by passage of potassium salt solution in water through a column of Dowex resin (H+ form). The elute was evaporated to dryness under reduced pressure [14].
All chemicals were obtained from commercial sources and used as received. Spin Coater, (S.C.S.86, Japan), Field Emission Scanning Electron Microscope (FESEM) (VEGA\\TESCAN-XMU, Czech Republic), Energy Dispersive Spectrometry (EDS) (Mira 3-XMU, Czech Republic), and UV–visible spectrophotometer, (Optizen UV3220, Germany) were used for characterization of samples. The average particle size of Au nanoparticles in the film was measured by SPSS software using Image J program.
Preparation of nanocomposite
Polyvinylalcohol (PVA) 30 wt% was dissolved in deionized water and stirred in an oil bath for 10 min. Then tetraethylorthosilicate 20 wt% and Wells–Dawson acid solutions were added. This mixture was refluxed at 353 K for 6 h and a clear viscous gel is formed. At the next stage, 250 µL of the obtained transparent gel was used to make films by spin coating method (500 rpm/min) and after reducing, it was dipped in different times including 5, 10, and 30 min into HAuCl4 solution (25 mL, 3 mM) [13].
Photodegradation experiments
The photo reactor was designed in our laboratory. In a typical reaction, a 250-mL Pyrex glass was equipped with a magnetic stirrer, azo dye solution, and nanocomposite film. The mixture was stirred and purged with nitrogen for 1 h, and then it was irradiated under the high-pressure mercury lamp (Philips, 125 W, wavelength 254 nm) as UV light source. The temperature in the glass reactor was set to 25 ± 2 °C by the circulating water. The experimental procedure is described as follows: a series of 10 mL solutions containing 30 mg L−1 of azo dye solution were prepared and then sample was placed under light irradiation in the presence of nanocomposite film. The progress of reaction was investigated using a UV–visible spectrophotometer.
Results and discussion
Heteropolyacids are well-established as powerful oxidants for the oxidation of a wide range of organic compounds. In this process, the excitation of the O → M charge transfer can occur under UV irradiation [10, 15, 16]. Interestingly, the reduced form of heteropolyacid can act as a strong reducing reagent which can be readily re-oxidized by various chemicals especially as metal ions [3, 15].
In this research, Wells–Dawson acid was used in synthesis of Au nanoparticles through a simple sol–gel and photoreduction process. In this process, Wells–Dawson acid played a dual role (a) photocatalytic reducing agent and (b) stabilizer [13].
The photoreduction of nanocomposite film was confirmed by UV–visible spectroscopy (Fig. 1). The formation of reduced Wells–Dawson in the composite film was confirmed by observation of deep blue color (Fig. 1). In UV–visible spectra (Fig. 1), the reduced Wells–Dawson acid showed absorption peaks at about 300 and 690 nm, which the observed peak in 690 nm is related to the formation of single electron-reduced Wells–Dawson ion. This figure also shows that there is an increase in the intensity up to 60 min, but after that, there is no change. This is a proof that all the Wells–Dawson ions in the composite have been reduced. The reduced film was used as reducing medium and host for the formation of Au nanoparticles [17].
With dipping of the reduced nanocomposite film with thickness 43 nm (Fig. 2) into HAuCl4 solution, a band at 530 nm was appeared which confirms the formation of Au nanoparticles in the composite film (Fig. 3). The formation of Au nanoparticles was further approved by changing the blue film to pink and violet color, respectively.
Figure 4 shows the FESEM images of reduced composite film contacted with HAuCl4 solution at different times. This figure shows when the time of dipping is increased from 5 to 30 min, the average particle size is also increased. The average size of nanoparticles is 10.54, 16.57, and 19.61 in Fig. 3a, b, d, respectively.
The EDS analysis is shown in Fig. 5. It shows the elemental composition of Au nanoparticles and Wells–Dawson in the generated nanofilms. The elemental constitution to control the loading amount of Au nanoparticles confirmed 9.91% w/w in the nanocomposite film. The quantitative results are shown in Tables 1 and 2.
Interestingly, comparison our results with the obtained results in preparation of Au nanoparticles using Preyssler HPA [13], H14[NaP5W30O110], showed a higher loading of gold nanoparticles (22%) under similar reaction conditions. Thus, the structure of heteropolyacid can affect the loading amount in this process. The difference can be attributed to the difference between oval and spherical structure of Preyssler and Dawson, respectively [18]. It is suggested that the larger number of H+ and W atoms in Preyssler may lower the activation barrier, and provides many “sites” on the oval-shaped structure. They are likely to render the loading effectiveness.
The catalytic activity of the synthesized nanocomposite film was studied in the decolorization of MeO and MR azo dyes. The results are reported in Figs. 6 and 7, and a comparison is reported in Table 3. A significant decrease in the absorbance bands can be observed with a decolorization degree of 96 and 87.2% after 36 and 43 min for MeO and MR, respectively.
The following equation was used to calculate the degree of azo dye decolorization [19–21]:
In this equation, C and C 0 are the decolorization degree and the initial absorbance of dyes solution, respectively. C e stands for the absorbance of the dye solution after photocatalysis. The pseudo-first order rate constants are given by the plot of ln(C/C 0) versus time.
Conclusions
Dawson acid, [H6P2W18O62], can be used as a superior and green-reducing reagent and stabilizer in the preparation of Au nanoparticles in a nanophotochromic film, via a simple and fast sol–gel procedure and photolysis. The synthesized nano film showed a significant catalytic activity in the decolorization process of MeO with pseudo-first order kinetic behavior.
Interestingly, our findings showed that heteropolyacid structure can control the loading amount of embedded Au nanoparticles up to twice. Further investigation in this field will provide a great opportunity for researches and scientists to develop techniques for the green and controlled synthesis of various nanoparticles in the presence of other heteropolyacids.
References
Ayati, A., Ahmadpour, A., Bamoharram, F.F., Tanhaei, B., Mänttäri, M., Lahtinen, M., Sillanpää, M.: Novel Au NPs/Preyssler acid/TiO2 nanocomposite for the photocatalytic removal of azo dye. Sep. Purif. Technol. 133, 415–420 (2014)
Mandal, S., Das, A., Srivastava, R., Sastry, M.: Keggin ion mediated synthesis of hydrophobized Pd nanoparticles for multifunctional catalysis. Langmuir 21(6), 2408–2413 (2005)
Wang, J., Lu, X., Fan, Sh, Zhao, W., Li, W.: In situ growth of gold nanoparticles on SiO2/lanthanide–polyoxometalates composite spheres: an efficient catalytic and luminescent system. J. Alloys Compd. 632, 87–93 (2015)
Mandal, S., Selvakannan, R.P., Pasricha, R., Sastry, M.: Keggin ions as UV-switchable reducing agents in the synthesis of Au core-Ag shell nanoparticles. J. Am. Chem. Soc. 125(28), 8440–8441 (2005)
Niu, C., Wu, Y., Wang, Z., Li, Z., Li, R.: Synthesis and shapes of gold nanoparticles by using transition metal monosubstituted heteropolyanions as photocatalysts and stabilizers. Front. Chem. China 4(1), 44–47 (2009)
Sadjadi, S., Heravi, M.M.: Recent advances in applications of POMs and their hybrids in catalysis. Curr. Org. Chem. 20(13), 1404–1444 (2016)
Heravi, M.M., Sadjadi, S.: Recent developments in use of heteropolyacids, their salts and polyoxometalates in organic synthesis. J. Iran. Chem. Soc. 6(1), 1–54 (2009)
Heravi, M.M., Bamoharram, F.F., Rajabzadeh, G., Seifi, N., Khatami, M.: Preyssler heteropolyacid [NaP5W30O110]14−, as a new, green, and recyclable catalyst for the synthesis of [1,2,4] triazino [4,3-b][1,2,4,5]tetrazines. J. Mol. Catal. A Chem. 25(1), 213–217 (2006)
He, T., Yao, J.: Photochromism in composite and hybrid materials based on transition-metal oxides and polyoxometalates. Prog. Mater. Sci. 51, 810–879 (2006)
Xing, X., Wang, M., Liu, R., Zhang, Sh, Zhang, K., Li, B., Zhang, G.: Highly efficient electrochemically driven water oxidation by graphene-supported mixed-valent Mn16-containing polyoxometalate. Green Energy Environ. 1, 138–143 (2016)
Zhang, Y., Bo, X., Nsabimana, A., Munyentwali, A., Han, C., Li, M., Guo, L.: Green and facile synthesis of an Au nanoparticles@polyoxometalate/ordered mesoporous carbon tri-component nanocomposite and its electrochemical applications. Biosens. Bioelectron. 66, 191–197 (2015)
Bamoharram, F.F.: Role of polyoxometalates as green compounds in recent developments of nanoscience. Synth. React. Inorg. Met. Org., Nano Met. Chem. 41(8), 893–922 (2011)
Rohani, N., Bamoharram, F.F., Marjani, A., Heravi, M.M.: A novel photochromic film based on Preyssler heteropolyacid and gold nanoparticles as a green and recyclable nanocatalyst for removal of azo dye from wastewaters. Curr. Nanosci. 12, 605–610 (2016)
Sheshmani, S., Arab, F.M., Mirzaei, M., Abedi Rad, B., Nouri, G.S., Yousefi, M.: Preparation, characterization and catalytic application of some polyoxometalates with Keggin, Wells–Dawson and Preyssler structures. Indian J. Chem. 50 A, 1725–1729 (2011)
Triantis, T., Troupis, A., Gkika, E., Alexakos, G., Boukos, N.: Photocatalytic synthesis of Se nanoparticles using polyoxometalates. Catal. Today 144(1–2), 2–6 (2009)
Liu, ChG, Zheng, T., Liu, Sh, Zhang, H.Y.: Photodegradation of malachite green dye catalyzed by Keggin-type polyoxometalates under Visible-light irradiation: transition metal substituted effects. J. Mol. Struct. 1110, 44–52 (2016)
Ayati, A., Tanhaei, B., Bamoharram, F.F., Ahmadpour, A., Maydannik, Ph, Sillanpää, M.: Photocatalytic degradation of nitrobenzene by gold nanoparticles decorated polyoxometalate immobilized TiO2 nanotubes. Sep. Purif. Technol. 171, 62–68 (2016)
Rohani, M., Bamoharram, F.F., Khosravi, M., Baharara, J., Heravi, M.M.: Preparation and characterization of Preyssler heteropolyacid-cellulose acetate hybrid nanofibers: a new, green and recyclable nanocatalyst for photodegradation of methyl orange as the model dye. J. Exp. Nanosci. 1–13 (2016). doi:10.1080/17458080.2016.1246754
Chen, Zh, Liao, J., Chen, Y., Zhang, J., Fan, W., Huang, Y.: Synthesis of oxygen deficient BiOI for photocatalytic degradation of methyl orange. Inorg. Chem. Commun. 74, 39–41 (2016)
Kumar, D.P., Reddy, N.L., Karthikeyan, M., Chinnaiah, N., Bramhaiah, V., Durga Kumari, V., Shankar, M.V.: Synergistic effect of nanocavities in anatase TiO2 nanobelts for photocatalytic degradation of methyl orange dye in aqueous solution. J. Colloid Interface Sci. 477, 201–208 (2016)
Ayati, A., Ahmadpour, A., Bamoharram, F.F., Heravi, M.M., Rashidi, H.: Photocatalytic synthesis of gold nanoparticles using Preyssler acid and their photocatalytic activity. Chin. J. Catal. 32(6), 978–982 (2011)
Acknowledgements
The authors would like to thank Research Center for Animal Development Applied Biology-Department of Nanobiotechnology, Mashhad Branch, Islamic Azad University, Mashhad, Iran and Department of Chemistry, Arak Branch, Islamic Azad University, Arak, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rohani, N., Bamoharram, F.F., Marjani, A. et al. Gold nanoparticles Wells–Dawson heteropolyacid nanocomposite film as an effective nanocatalyst in photocatalytic removal of azo dyes from wastewaters. J Nanostruct Chem 7, 171–178 (2017). https://doi.org/10.1007/s40097-017-0218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-017-0218-5