Abstract
The synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones, (3) has been achieved via the reaction of 5-amino-1-phenyl-1H-pyrazolo-4-carboxamide 1 with aromatic aldehyde in the presence of acidic cesium salt of Preyssler as a green and reusable nanocatalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyoxometalates (POMs) have attracted much attention as building blocks for functional composite materials due to their interesting nanosized structures [1].They are superlative models for the construction of hybrid systems, hence they are considered as the potential candidates to be converted into the nanometer-sized materials. In recent years, considerable effort has been devoted to the design and controlled fabrication of nanostructured POMs for being used in green reactions. These waves of interest have resulted in the development of numerous strategies for the synthesis of nanostructured materials in a wide range of sizes. Therefore, the subject of nano POMs and their applications continues to attract dramatic attentions and the number of publications and patents continues to grow day to day, and new researchers are attracted to the field. Thus, plenty of room exists for extending the exploration of these materials and further expansion of new POMs is still in much demands. However, in spite of extensive investigations on using of Keggin-type heteropolyacids in nanotechnology [2, 3], the role of nano Preyssler has been largely overlooked. Recently, we have reported the application of Preyssler catalyst, H14[NaP5W30O110], in various fields of nanotechnology [4–9]. In our attempts to use Preyssler as catalyst in organic reactions, we reported that Preyssler-type heteropolyacid, H14 [NaP5W30O110], shows good catalytic activity in the synthesis of heterocyclic compounds [10–14]. Encouraged by these experiences, it was of great interest using Preyssler’s anion in nanoform in the synthesis of heterocyclic compounds.
Herein, in continuation of our interest in catalytic applications of H14 [NaP5W30O110] in the synthesis of heterocyclic compounds [10–14] and extending the applications of this catalyst in nanotechnology, and because of importance of Pyrazolo[3,4-d]pyrimidines in medicine as anti-tumor, and anti-leukemia activities [15–19], we wish to report the synthesis of some derivatives of 1H-pyrazolo[3,4-d]pyrimidin-4(5H)-ones, by acidic cesium salt of Preyssler nanoparticles, Cs12H2 [NaP5W30O110], as a green and eco-friendly solid nanocatalyst. In general, acid-catalyzed condensation of 5-amino-1-phenyl-1H-pyrazolo-4-carboxamide 1 with aromatic aldehydes and carboxylic acids is one of the simplest and most applicable approaches for synthesis of Pyrazolo[3,4-d]pyrimidines. Veeranagaiah and co-workers [20] have also used acetic acid or hydrochloric acid and poly phosphoric acid or poly phosphate ester as an acid catalyst. These catalysts are toxic and dangerous and the applications of them cause environmental problems. The mentioned reactions were carried out in refluxing acetic acid during 2–4 h in the presence of hydrochloric acid and 5 h refluxing in acetic acid in the absence of catalyst in 47–60 % yields [20].
Our findings indicate that our green used nanocatalyst is effective for synthesis of 1H-pyrazolo[3,4-d]pyrimidin-4(5H)-ones in excellent yields.
Experimental
Chemicals and instruments
All of the chemicals were purchased from Merck and Sigma Aldrich Companies and used as received. FT-IR spectra were recorded with a Brucker scientific spectrometer (solid sample, KBr pellets). The synthesized nanostructures were characterized by Transmission Electron Microscopy (PHILIPS CM-120). The Cs concentration was analyzed by an inductively coupled plasma optical atomic emission spectrometry (ICP-OES) model iCAP 6300 (Thermo Electron Corporation). The specific surface area (SBET) of catalyst was determined by nitrogen adsorption/desorption techniques. 1H NMR spectra were recorded on a 100 MHz spectrometer using TMS as internal standard. Mass spectra were obtained from Varian CH-7 instrument at 70 eV. The Preyssler’s anion [NaP5W30O110]14− was prepared according to our previous method [5].
For preparation of nano Cs-Preyssler, Preyssler acid and CsCl in a mole ratio of 1:14 were put into a mortar and several drops of surfactant Triton X-100 were added. The mixture was ground for 50 min and after washing in a supersonic washing machine, the mixture centrifuged. The synthesized nanoparticles dried in an oven for 4 h (50–60 °C). The number of Cs ions obtained by ICP measurement and titration method was 12.
General procedure for the synthesis 6-substituted pyrazolo[3,4-d]pyrimidin-4(5H)-ones
A solution of 1 (0.9 mmol) and appropriate aromatic aldehyde 2 (0.9 mmol) and the nanocatalyst in acetic acid was refluxed for 1–3 h. The catalyst was removed by filtering and washed. The nanocatalyst can be easily separated by filtration and can be recycled in reaction. After four times, the yields decreased only by 4 %. The filtrate was cooled and the solid was filtered, washed with water, dried and re-crystallized in ethanol to give pure product. All compounds were characterized by mass, IR and 1H NMR spectra (Table 2).
Results and discussions
Recently, we described synthesis of some new derivatives of 1H-pyrazolo[3,4-d]pyrimidin-4(5H)-ones by heteropolyacids such as H3[PW12O40] and H14[NaP5W30O110] as acidic catalysts instead of mineral acids like HCl or polyphosphoric acid as a more eco-friendly and suitable method [21, 22]. Therefore, it is of great interest to know, what occurs if we check Preyssler nanocatalyst in these reactions. Also, another goal in this research was an answer to this question: Do surface area in the Preyssler structure, which is responsible for surface type reactions has an important role on yields?
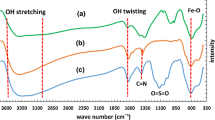
In the preparation of nanoparticles of Cs12H2 [NaP5W30O110], to balance anions charge, the stoichiometric amounts of aqueous solutions of cations were used (1:14). However, due to the charge and size differences between Cs+ and H+ ions, different amounts of cations appeared in the Preyssler and the rest of charges were compensated for by protons to form acid salts. The number of Cs ions obtained was 12, by ICP measurement and titration method. Figure 1 shows the FT-IR spectrum of the Cs12H2 [NaP5W30O110]. In the nano Cs-Preyssler, the Preyssler’s anion displays vibrations at 1161.96, 1087.62, and 1023.33 cm−1 for P–O stretching, 956.60 and 907.76 cm−1 for W–O–W stretching, and 772.78 cm−1 for W=O stretching.
From Fig. 1, it can be seen that the nano Cs-Preyssler shows all the IR vibration peaks assigned to a Preyssler structure, and the locations of featured peaks are in well agreement with its structure [23]. TEM image of nano Cs-Preyssler is shown in Fig. 2. The TEM image shows the spherical structures which have a diameter in the range of 5–25 nm.
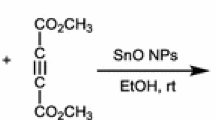
When the mixture of 5-amino-1-phenyl-1H-pyrazolo-4-carboxamide 1 and aromatic aldehyde 2 in acetic acid were refluxed in the presence of catalytic amounts of mentioned nanoheteropolyacid for 1–3 h, 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones 3 were obtained (Scheme 1).
The results are shown in Tables 1 and 2. No other by products were recognized.
In spite of obtaining good to moderate yields of products in our earlier works [21, 22], we obtained higher yields using nano Preyssler catalyst (Table 1). It is suggested that, as the particle size decreases, the surface area increases, and thus activity increases. The larger surface area of cesium nano Preyssler in comparison with pure Preyssler acid was confirmed by BET analysis. The BET surface area of Cs12H2[NaP5W30O110] and H14[NaP5W30O110] obtained was 2.61 and 0.78 m2/g, respectively. Surface-type and bulk-type catalysis are the two types of catalysis for heterogeneous acid catalysis by heteropolyacids [24, 25]. In surface type catalysis, the reactions occur on the surface of bulk or supported heteropoly compounds and the catalytic activity usually depends on the surface acidity of heteropolyacid. We believe that the larger surface area in nano Cs12H2[NaP5W30O110] is responsible for absorbing a greater number of reactant molecules.
The selectivity toward 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones remained 100 % in all cases.
Conclusion
We reported here a catalytic method for synthesis of the 6-substituted-pyrazolo[3,4-d]pyrimidin-4(5H)-ones using acidic cesium salt of Preyssler nanoparticles which is found as a more effective catalyst than the other heteropolyacids in our earlier work as well as classical acids.
This nanocatalyst is not soluble in acetic acid and is eligible to an easy separation and recovery by filtering for their further reuse without any appreciable decrease of the catalyst activity.
References
Bamoharram, F.F.: Role of polyoxometalates as green compounds in recent developments of nanoscience. Synth. React. Inorg., Met.-Org., Nano Met. Chem. 41, 893–922 (2011)
Sawant, D.P., Vinu, A., Jacob, N.E., Lefebvre, F., Halligudi, S.B.: Formation of nanosized zirconia supported 12-tungstophosphoric acid in mesoporous silica SBA-15: a stable and versatile solid acid catalyst for benzylation of phenol. J. Catal. 235, 341–352 (2005)
Uchida, S., Mizuno, N.: Design and syntheses of nano-structured ionic crystals with selective sorption properties. Coordin. Chem. Rev. 251, 2537–2546 (2007)
Bamoharram, F.F., Heravi, M.M., Roushani, M., Toosi, M.: Synthesis and characterization of silica-supported Preyssler nanoparticles and its catalytic activity for photodegradation of methyl orange. Green. Chem. Lett. Rev. 2, 35–41 (2009)
Bamoharram, F.F., Heravi, M.M., Saneinezhad, S., Ayati, A.: Synthesis of a nanoorgano-silicon compound for building materials waterproofing, using heteropolyacids as a green and eco-friendly catalyst. Prog. Org. Coat. 76, 384–387 (2013)
Rashidi, H., Ahmadpour, A., Bamoharram, F.F., Heravi, M.M., Ayati, A.: The novel, one step and facile synthesis of ZnO nanoparticles using heteropolyoxometalates and their photoluminescence behavior. Adv. Powder Technol. 24, 549–553 (2013)
Bamoharram, F.F., Heravi, M.M., Heravi, M.M., Meraji, M.: Synthesis of silver nanoparticles in the presence of a green heteropolyacid, H14[NaP5W30O110], and their catalytic activity for photo degradation of methylen blue and methyl orange. Int. J. Green Nanotechnol. Phys. Chem. 1, 26–31 (2009)
Ayati, A., Ahmadpour, A., Bamoharram, F.F., Heravi, M.M.: A green and simple route for the controlled-size synthesis of gold nanoparticles using Preyssler heteropolyacid. Synth. React. Inorg. Met Org. Nano Met. Chem. 42, 1309–1314 (2012)
Bamoharram, F.F., Ahmadpour, A., Heravi, M.M.: Synthesis of carbon nanotubes via catalytic chemical vapor deposition method and their modification with Preyssler anion, [NaP5W30O110]14−. NANO 6, 349–355 (2011)
Tavakoli-Hoseini, N., Heravi, M.M., Bamoharram, F.F., Davoodnia, A., Ghassemzadeh, M.: An unexpected tetracyclic product isolated during the synthesis of biscoumarins catalyzed by [MIM(CH2)4SO3H][HSO4]: characterization and X-ray crystal structure of 7-(2-hydroxy-4-oxo-4H-chromen-3-yl)-6H,7H-chromeno[4,3-b]chromen-6-one. J. Mol. Liq. 163, 122–127 (2011)
Heravi, M.M., Sadjadi, S., Mokhtari Haj, N., Oskooie, H.A., HekmatShoar, R., Bamoharram, F.F.: A novel multi-component synthesis of 4-arylaminoquinazolines. Tetrahedron Lett. 50, 943–945 (2009)
Heravi, M.M., Sadjadi, S., Sadjadi, S., Oskooie, H.A., Bamoharram, F.F.: A convenient synthesis of bis(indolyl)alkanes under ultrasonic irradiation using silica-supported Preyssler nano particles. Ultrason. Sonochem. 16, 718–720 (2009)
Heravi, M.M., AlimadadiJani, B., Derikvand, F., Bamoharram, F.F., Oskooie, H.A.: Three component, one-pot synthesis of dihydropyrano[3,2-c]chromenederivatives in the presence of H6P2W18O62-18H2O as a green and recyclable catalyst. Catal. Commun. 10, 272–275 (2008)
Heravi, M.M., Ranjbar, L., Derikvand, F., Alimadadi, B., Oskooie, H.A., Bamoharram, F.F.: A three component one-pot procedure for the synthesis of [1,2,4]triazolo/benzimidazolo-quinazolinone derivatives in the presence of H6P2W18O62·18H2O as a green and reusable. Catal. Mol. Divers. 12, 181–185 (2008)
Cottam, H.B., Mackernan, P.A., Robins, P.A., Revanker, R.K.: Synthesis and biological activity of 6-azacadeguomycin and certain 3,4,6-trisubstituted pyrazolo[3,4-d]pyrimidine ribonucleosides. J. Med. Chem. 28, 1010–1016 (1985)
Bhat, G.A., Montero, J.G., Panzica, R.P., Worting, L.L., Towsend, L.B.: Pyrazolopyrimidine nucleosides. 12. Synthesis and biological activity of certain pyrazolo[3,4-d]pyrimidine nucleosides related to adenosine. J. Med. Chem. 24, 1165–1172 (1981)
Zacharie, B., Connolly, T.P., Attardo, R., Penney, G.: A short synthesis of 4-substituted 1-(hydroxyalkyl)-1H-pyrazolo[3,4-d]pyrimidines. Tetrahedron 52, 2271–2278 (1996)
Andrson, J.D., Cottom, H.B., Larson, S.B., Nord, L.D., Revankar, G.R., Robins, R.K.: Synthesis of certain pyrazolo[3,4-d]pyrimidin-3-one nucleosides. J. Heterocyclic. Chem. 27, 439–453 (1990)
Avila, J.L., Polegre, M.A., Avila, A.R., Robins, K.: Action of pyrazolopyrimidine derivatives on American Leishmania spp. promastigotes. Comp. Biochem. Physicol. C. 83, 285–289 (1986)
Reddy, K.H., Reddy, A.P., Veeranagaiah, V.: Synthesis of alkyl/aryl substituted pyrazolo[1,5-a]pyrimidines. Ind. J. Chem. B. 31, 163–166 (1992)
Heravi, M.M., Motamedi, R., Seifi, N., Bamoharram, F.F.: Catalytic synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacid: H14[NaP5W30O110] and H3PW12O40. J. Mol. Catal. A: Chem. 249, 1–3 (2006)
Heravi, M.M., Motamedi, R., Bamoharram, F.F., Seify, N.: A catalytic method for synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacids:H14[NaP5W29MoO110] and H3PMo12O40. Catal. Commun. 8, 1467–1471 (2007)
Alizadeh, M.H., Harmalker, S.P., Jeannin, Y., Martin-Frere, J., Pope, M.T.: A heteropolyanion with fivefold molecular symmetry that contains a nonlabile encapsulated sodium ion. The structure and chemistry of [NaP5W30O110]14−. J. Am. Chem. Soc. 107, 2662–2669 (1985)
Okuhara, T., Mizuno, N., Misono, M.: Catalytic chemistry of heteropoly compounds. Adv. Catal. 41, 113–252 (1996)
Misono, M.: Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 13, 1141–1152 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bamoharram, F.F., Heravi, M.M., Ayati, A. et al. Acidic cesium salt of Preyssler nanoparticles: a new, green and recyclable nanocatalyst for the synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones. J Nanostruct Chem 4, 93 (2014). https://doi.org/10.1007/s40097-014-0093-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40097-014-0093-2