Abstract
The properties of L10-FePt nanoparticles can be improved in the presence of MgO and Ag interlayers in direct sputtering and annealing method, respectively. Such properties are crystal and compound ordering, nanostructure and crystal orientation. In this work, FePt nanoparticles in ferromagnetic L10-fct phase were synthesized using sputtering method on Ag and MgO layers. According to XRD analyses, the impact of the presence of these two kinds of interlayer on crystal structure and its orientation has been investigated. Furthermore, the effect of the presence of 10% Ag on these properties has been studied and their granular layer nanostructures were characterized through the FE-SEM analysis. The results show that the presence of Ag as nanocompound and interlayer is desirable on declining the transition temperature and controlling the size during annealing. The presence of MgO as a sublayer in direct synthesis leads to the formation of 10 nm monosize smaller nanoparticles. According to VSM analysis, MgO and Ag sublayers have increased the magnetic coercivity of the FePt nanoparticles by 3.4 times and 3.7 times, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FePt nanoparticles in L10-fct compound ordering phase hold scientists’ attention so as to their magnetic anisotropy and application in magnetic recording of data. Formation of this phase requires high temperature with control in the size of nanoparticles. L10-fct anisotropic ferromagnetic nanoparticles can be synthesized using sputtering method at 500 °C [1, 2]. And also, it is possible that deposition is done at room temperature following which samples are annealed at temperature higher than 550 °C [3, 4]. The L10-fct crystal structure needs to be formed with control in the size of nanoparticles. The presence of Ag as a sublayer [5, 6] and multilayer leads to a decrease in L10-fct transition phase temperature [7]. This is the effect on the decline of the obtained grains’ size [8]. The other impact of Ag is making crystal direction of nanoparticles aligned, as a thin film on the Ag surface [9, 10]. Therefore, the presence of Ag is useful for nanostructural and compound ordering of FePt up to roughly 10% Ag [6].
The growth of nanometric FePt layers on MgO layer [11, 12] gives rise to controlling the size and making these nanoparticles orient crystally [13] in L10-fct compound ordering phase. The surface of MgO is uniform with tiny grains, and the aligned crystal growth of FePt nanoparticles on it is possible [14]. Choosing appropriate percentage of Ag using Co sputtering [6], it can be possible to provide a uniform distribution of FePt nanoparticles [6].
Fe/Pt/Fe is aligned with (001) crystal direction after annealing at 673 °C [13]. This occurs at 600 °C of annealing temperature in the case of FePt/Ag/SiO2 [9] and at annealing temperature of 550 °C for [FePt/Ag]n multilayers [7]. Moreover, the presence of Ag with deposition at 550 °C leads to the increase in magnetic coercivity up to the order of Tesla [4], but for deposition at high temperature or annealing in Ag presence, there is not any suitable report about nanostructure. Ag layers in Ag/FePt/Ag contribute to the increase in FePt grain’s size at 700 °C up to the 83 nm on average [15].
MgO single-crystal substrate in (100) direction leads to the directional growth of FePt/MgO [11] and FePt/Pt/MgO [16]. This increases coercivity up to the order of Tesla, but needs MgO(100) single-crystal substrate.
In this work, samples of FePt/Ag/Si and FePt/MgO/Si layers were synthesized using sputtering on hot substrate as well as a two-step synthesis: sputtering at room temperature and then annealing 90% Ar–10% H2 atmosphere at 600 °C. More importantly, the impact of sublayer on magnetic properties, their crystal and nanocrystal structures was determined and compared with each other.
Experimental details
First, Ag and MgO nanolayers were deposited on (001) Si wafers using sputtering method. Then, FePt nanoparticles with 10% Ag were deposited on synthesized Ag and MgO layers using rf and dc sputtering methods, respectively. The base vacuum pressure has been \(4 \times 10^{ - 5} \,{\text{torr}}\), and Ar pressure has been \(2 \times 10^{ - 2} \,{\text{torr}}\). A group of samples were synthesized at room temperature and then annealed at 500 °C and 600 °C for 60 min in 90% Ar–10% H2 atmosphere. Another group of samples were synthesized on Ag/Si and MgO/Si layers at 500 °C using sputtering method. The substrate holder is heated by an electric heater, and the cathode level is 7 cm above the substrate. The synthesis conditions are shown in Table 1 for comparison.
In order to determine the crystal structure, compound ordering and crystal orientation of the nanoparticles, XRD analysis was used in STOE STADI MP model with the wavelength of 1.54 Å. The magnetic properties of the samples were investigated using a vibrating sample magnetometer, VSM, Lake-Shore model 7400 with the maximum field up to 20 kOe. Conventional SEM studies were carried out using a FE-SEM model MIRA3 TESCAN working at 15.0 kV.
Results and discussion
Figure 1 shows the results of the analysis of FePt nanoparticles on a layer of Ag, which were annealed after depositing by sputtering. Figure 1a shows a granular layer of Ag with the average thickness of 10 nm that has been deposited on Si wafer at room temperature. It is considered that Ag has grown granularly and formed discontinuous background. Figure 1b–d shows sample 1. Figure 1b shows a 20-nm layer of FePt which was deposited at room temperature on a 40-nm Ag layer. Then the sample was annealed at 500 °C (Fig. 1c) and 600 °C (Fig. 1d) for 1 h. As the presence of Ag sublayer makes it possible for FePt nanoparticles to transit to L10-fct phase at 500 °C, Fig. 1c shows a kind of suitable situations in which a relatively uniform nanostructure is obtained. At temperatures above 600 °C, Ag atoms are separated from the sublayer and constitute smaller Ag nanoparticles (Fig. 1d) and FePt layer has lost its uniformity. The image of sample 2 in comparison with sample 3 is shown in Fig. 1e, f. The presence of Ag sublayer in direct synthesis with sputtering method at 500 °C, which forms larger and uniform growth, is not suitable (Fig. 1e). Therefore, Ag interlayer presence is just suitable in indirect synthesis, nanostructurally (Fig. 1c).
In Fig. 2 the effect of MgO sublayer in samples 5 and 6 is shown using FE-SEM analysis. Figure 2a shows a 10-nm uniform MgO granular layer which has been deposited on Si using magnetosputtering method. Figure 2b–d shows sample 5 which is FePt–Ag/MgO/Si with 10% Ag that has been deposited at room temperature and has not been annealed (Fig. 2b) and has been annealed at 500 °C (Fig. 2c) and 600 °C (Fig. 2d). At high temperature, the absence of MgO bonding with Si sublayer leads to the formation of FePt–Ag/MgO islands on Si and separately tiny Ag nanoparticles. It has become obvious that MgO sublayer is not suitable in annealing stage. However, according to Fig. 2e, in direct synthesis with magnetosputtering method at 500 °C the MgO sublayer presence gives rise to the uniformity and appropriate size of FePt–Ag (sample 6). It is obtained from the comparison of Fig. 2e with Fig. 2f. Figure 2f shows the image of sample 7 which is without MgO layer. Therefore, the presence of MgO interlayer is nanostructurally suitable just in indirect synthesis. In direct synthesis method, the presence of MgO layer is useful, and in annealing after deposition at room temperature method, the presence of Ag layer is suitable.
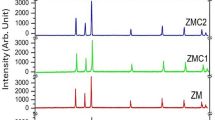
XRD patterns of three samples, 7, 5c and 1c which were annealed without sublayer, with MgO and Ag sublayers, respectively, are shown in Fig. 3a–c. The presence of MgO and Ag layers leads to an increase in the (001) peak intensity in L10-fct structure (Fig. 3b, c). The presence of (001) peak in FePt structure is the evidence for the transition to L10-fct phase with compound ordering, and the (001) to (111) peak intensity ratio is an indicator for directional growth of nanoparticles in mentioned phase in (001) direction perpendicular to the surface of the layer.
The separation of (002) and (200) peaks in Fig. 3c shows that the structure converted from (fcc) to (fct). The higher (002) peak intensity with respect to (002) or the elimination of (200) peak is another confirmation for samples to be directional due to the presence of MgO and Ag interlayers or Ag as a nanocomposite, which is shown in Fig. 3.
Figure 4 shows XRD analyses of FePt samples which were deposited at 500 °C with 10 nm thickness using direct synthesis method. In Fig. 4b, c MgO and Ag interlayers are used, respectively, and Fig. 4a shows the sample deposited without any sublayer on Si wafer. In these figures, every condition is the same showing the type of sublayer. The presence of MgO or Ag gives rise to the appearance of L10 compound ordering peaks, such as (001) and (110). Figure 4a lacks these two peaks because this sample lacks MgO or Ag interlayer.
The result of VSM analyses of samples 2, 3 and 4 which were synthesized using direct sputtering method on hot substrate is shown in Fig. 5.
Figure 5a shows FePt/Si sample which lacks interlayer. Figure 5b, c shows FePt/MgO/Si and FePt/Ag/Si, respectively. The effect of the presence of MgO and Ag sublayers was increased by 3.4 and 3.7 times, respectively, in coercivity.
Conclusion
Ag presence as the nanocomposite or an interlayer in FePt nanoparticles accounts for a decline in L10-fct compound ordering phase transition temperature of these nanoparticles as well as alignment of their c crystal axis vertical to the surface of the layer. This alignment would also be possible in the presence of MgO interlayer. This layer in direct synthesis at 500 °C leads to the formation of smaller and monosize FePt nanoparticles, and the presence of 10% Ag helps to form L10-fct phase. Ag interlayer in annealing process, so as to L10-fct phase transition, is a desirable impact on uniformity of nanostructure in as much as this makes phase transition at 500 °C annealing temperature possible. Both Ag and MgO interlayers bring about an increase in magnetic coercivity of FePt nanoparticles.
References
Chen, J.S., Zhou, T.J., Ding, Y.F., Lim, B.C., Liu, B.: Microstructure and magnetic properties of rapidly annealed FePt (001) and FePt–MgO (001) films. J. Appl. Phys. 97(10), 10N108 (2005)
Sheikhi, M., Sebt, S.A., Khajehnezhad, A.: The Effect of deposition rate on FePt/MgO crystal orientation. Physica C 549, 37–39 (2018)
Ohtake, M., Itabashi, A., Kirino, F., Futamoto, M.: L10 Ordered FePd, FePt, and CoPt thin films with flat surfaces prepared on MgO (110) single-crystal substrates. IEEE Trans. Magn. 49(7), 3295–3298 (2013)
Xu, X.H., Wu, H.S., Wang, F., Li, X.L.: Structure and magnetic properties of FePt and FePt/Ag thin films deposited by magnetron sputtering. Thin Solid Films 472(1–2), 222–226 (2005)
Xu, Y., Sun, Z.G., Qiang, Y., Sellmyer, D.J.: Magnetic properties of L10 FePt and FePt:Ag nanocluster films. J. Appl. Phys. 93(10), 8289–8291 (2003)
Roghani, R., Sebt, S.A., Khajehnezhad, A.: High magnetic coercivity of FePt–Ag/MgO granular nanolayers. Physica C 549, 15–17 (2018)
Wang, F., Xu, X., Wu, H.: Magnetron sputtering deposition of [FePt/Ag]n multilayers for perpendicular recording. Rare Met. 25(1), 47–50 (2006)
Wei, D.H., Yuan, F.T., Chang, H.W., You, K.L., Lieu, Y., Yao, Y.D., Wu, J.K.: Magnetization reversal and microstructure of FePt–Ag (001) particulate thin films for perpendicular magnetic recording media. J. Appl. Phys. 103, 07E116 (2008)
Wang, H., Zhang, J., Mo, Q., Yang, F.J., Wang, H.B., Miao, X.S.: Microstructure and magnetic properties of (001)-oriented L10 FePt films: role of Ag underlayer and Fe/Pt ratio. Mater. Res. Bull. 47(10), 2974–2976 (2012)
Varaprasad, B.C.S., Takahashi, Y.K., Hono, K.: Microstructure control of L10-ordered FePt granular film for heat-assisted magnetic recording (HAMR) media. JOM 65(7), 853–861 (2013)
Weisheit, M., Schultz, L., Fähler, S.: Temperature dependence of FePt thin film growth on MgO (100). Thin Solid Films 515(7–8), 3952–3955 (2007)
Yu, M., Ohguchi, H., Zambano, A., Takeuchi, I., Liu, J.P., Josell, D., Bendersky, L.A.: Orientation and magnetic properties of FePt and CoPt films grown on MgO (110) single-crystal substrate by electron-beam coevaporation. Mater. Sci. Eng. B 142(2–3), 139–143 (2007)
Vladymyrskyi, I.A., Karpets, M.V., Katona, G.L., Beke, D.L., Sidorenko, S.I., Nagata, T., Nabatame, T., Chikyow, T., Ganss, F., Beddies, G., Albrecht, M.: Influence of the substrate choice on the L10 phase formation of post-annealed Pt/Fe and Pt/Ag/Fe thin films. J. Appl. Phys. 116(4), 044310 (2014)
Shiroyama, T., Varaprasad, B.C.S., Takahashi, Y.K., Hono, K.: Influence of MgO underlayers on the structure and magnetic properties of FePt-C nanogranular films for heat-assisted magnetic recording media. AIP Adv. 6(10), 105105 (2016)
Tsai, J.-L., Lin, G.-B., Tzeng, H.-T.: Magnetic properties and microstructure of (0 0 1) oriented Ag/FePt, Ag/FePt/Ag films. J. Alloys Compd. 487(1–2), 18–23 (2009)
Kim, M.G., Shin, S.C.: Temperature dependence of growth morphology of sputtered (FePt/Pt) films on MgO (100) substrate. J. Appl. Phys. 90(5), 2211–2215 (2001)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sheikhi, M., Sebt, S.A. & Khajehnezhad, A. Comparing the structural and magnetic properties of FePt/MgO/Si and FePt/Ag/Si granular nanolayers. J Theor Appl Phys 13, 101–106 (2019). https://doi.org/10.1007/s40094-019-0328-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-019-0328-1