Abstract
(CuCl)4−2x−(KCl)x–CdCl2, x = 0.0–0.4 solid electrolytes were grown via solid-state reaction procedure by suitable heat treatment. An AC impedance spectroscopy suggested that the ionic conductivity mainly arisen from grain effect. A DC electrical conductivity of 3.94 × 10−5 Scm−1 was measured for the x = 0.3 composition at 320 °C. This also shows lowest activation energy in the temperature range of 293–593 K. This has been explained by that fact that at high temperatures thermal disturbances through lattice vibrations take place. In such a high-temperature region, Cu+ readily jumps and migrates at short range, but the number of mutual collisions also increases resulting in a decrease of the Cu+ mobility. The present study reveals that the change in conductivity value depends on concentration of doped ingredient as well as on various parameters in the system. Therefore, these solid electrolytes will be suitable for the development of different electrochemical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Superionic materials, which permit ions to move quickly and freely through their crystal lattice, possess unusually high level of ionic conductivity approximately 10−3 to 10−1 Scm−1 at somewhat moderate (400–700 °C) temperatures [1]. Recently, the act of mobility of large basic cationic structure in the form of tiny (fine porous) materials has been used to persuade substantial ionic conductivity [2]. In the past, enormous research work on crystalline solid electrolytes like, for example, Nasicon and Lisicon [3,4,5], Bimivox [6, 7], and rock salts [8] has resulted in developing several high-performance ionic conductors. The ionic conductivity of solid electrolytes with various cations such as H+ [9], Li+ [10], Na+ [11], K+ [12], Rb+ [13], Cs+ [14], Cu+ [15], Ag+ [16], Tl+ [17], Sn2+ [18], Sr2+ [19] and different anions such as O2− [20, 21] and F− [22] has been fruitfully utilized in the past few decades. Researchers observed approximately 0.3 Scm−1 conductivity in Ag and Cu halide systems at room temperature [23, 24]. Further binary halide systems of lithium (e.g. LiI–RbI [25]) have been studied for high ionic conductivity. Fast ion conductors with high copper ionic conductivity and low electronic conductivity have been noticed in the binary chloride (RbCl–CuCl) system [26,27,28]. In this work, we have been interested in KCl-doped ternary system (CuCl)4−2x–(KCl)x–CdCl2, x = 0.0–0.4 as possible ionic conducting materials, and an orthorhombic structural type represented by the compound CuCdCl3 has been prepared. Furthermore, the sample with x = 0.3 composition displays high ionic conductivity (3.94 × 10−5 Scm−1 at 320 °C). The AC and DC electrical properties of the CuCl–KCl–CdCl2 ionic conducting system by managing the (CuCl)4−2x–(KCl)x–CdCl2, x = 0.0–0.4 content in the samples are offered. The electrical properties of samples were characterized by modern analytical techniques. Room-temperature X-ray analysis and differential scanning calorimetry analysis of the pure compound, CuCdCl3, have been discussed before [29].
In the CuCl–KCl–CdCl2 ionic conducting system, the main compound which is under examination is copper (I) chloride, which has high symmetry of fcc cubic lattice structure and a substantial ionic nature. Besides that, CuCl, like all other copper (I) halides, is designated by intrinsic Frenkel defects (cationic vacancies and completely ionized interstitial cations) [30]. However, it may be mentioned that CuCl at low temperatures possesses a high level of electronic conduction.
The part portrayed by the doping ingredient increases, which may affect the nature of conduction of a ternary system as a whole to a notable degree. In the case of cationic disordering, the work done by the doping material such as an anion and a cation of a higher valence comes out to be efficient [31]. With the help of a quasi-chemical reaction (Eq. 1), a possible defect formation mechanism may be explained as,
“Me ·Cu ” positively charged ionic defects may act as electron traps, which are capable of decreasing the electronic conduction in the system which arises in the form of perfect ionic conduction of the CuCl–KCl–CdCl2 ternary system. The availability of a significant proportion of vacancies in the copper sublattice generates a new possibility for the occurrence of mono-polar ionic conduction by copper. We can notice the quantity of ionic defects by varying the composition of the doping compound, which on the other hand explains the involvement and extent of ionic conductivity in a system. An interstitial hopping process of K+ in a primarily CuCl lattice is responsible for the enhancement of ionic conductivity in the ionic system [32].
Experimental section
Preparation of solid electrolytes
(CuCl)4−2x–(KCl)x–CdCl2 (x = 0.0, 0.1, 0.2, 0.3, and 0.4) solid samples were fabricated via solid-state reaction using CuCl, KCl, and CdCl2 powders at a temperature of 400 °C. The required mixed proportion of powders was first grinded for 4 h using agate motor and a pestle with acetone and dried at 100 °C for 2 h, followed by another 4 h of grinding. Circular pellets were obtained by hydraulic pressing of the powders at 100 Mpa using stainless steel die. Opposite surfaces of all the pelletized samples with approximate dimensions of 0.65 cm radius and 0.1 cm thickness were coated with carbon and annealed between the electrodes at 100 °C for 2 h to ensure large electrical contact and to eliminate the grain boundary resistance between the samples and the electrodes. Then, they were kept in a silica gel desiccant contained desiccator for further use.
Electrical characterization
The electrical properties were characterized by demonstrating impedance spectrum and conductivity of solid samples. Alternating current (AC) impedance measurements were undertaken using a WAYNE KERR LCR METER-4300 in the frequency range of 10 Hz to 1 MHz at an amplitude of 1 V at 300 °C. The sample was kept at the desired temperature for an hour before each measurement. The conductivity parameters of KCl-doped solid electrolytes were evaluated under varying range of temperature (20–320 °C) at a single frequency of 1 MHz and at frequency range of 10 kHz–1 MHz at 35 °C using the above WAYNE KERR-4300 impedance analyser.
Results and discussion
Thermal and X-ray data
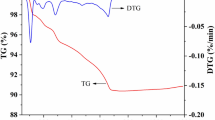
DSC and X-ray diffraction of the pure compound were discussed by Titilayo AK in 1987 [29]. The author reported endothermic peaks at 90, 125, 290, 323, and 440 °C. The peaks at 90 and 125 °C are due to the dehydration of the sample resulting in a possible phase change which is evident by the peaks at 290 and 323 °C, while the peak at 440 °C is due to the melting of the compound.
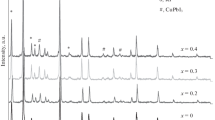
Room-temperature X-ray analysis of freshly prepared hydrated samples shows that the material is orthorhombic with lattice constants ao = 8.74 Å, bo = 13.95 Å, and co = 5.23 Å. However, X-ray analysis of powder samples initially annealed at 250 °C for 2 h shows tetragonal structure of the compound with lattice constants a = 5.25 Å and c = 8.18 Å. Therefore, dehydration process between 90 and 125 °C can lead to a phase change from orthorhombic to a tetragonal phase.
Impedance analysis
Impedance spectroscopy (IS) was used to find out the electrical conductivity. Figure 1 shows the impedance spectra of (CuCl)4−2x–(KCl)x–CdCl2 samples at 300 °C. All spectra exhibit a semicircle at high to medium frequencies and a small spike at low frequencies. Bulk and grain boundary resistance is evident from the semicircle, and a spike can be correlated with the Cu-ion transfer resistance between the electrode and the electrolyte. This points out that the conductivity is mainly due to the motion of ion vacancies (or ionic in nature) [33, 34]. It can be seen that the sample with x = 0.3 composition of KCl shows the smallest semicircle diameter and, therefore, the lowest total resistance. The value of total ionic conductivity (σtotal) of the samples can be calculated by the relation, σ = l/RtA, where l and A are thickness and surface area of the pellet, respectively. With increasing dopant content from 0.0 to 0.3 per formula unit, the conductivity increases and then starts to decrease. The increment in conductivity may be because of the structural change undertaken upon K doping and availability of higher Cu+ concentration, whereas the decrease in conductivity at higher K concentrations is because of the occurrence of low conductive phase and a decline in the availability of vacancies.
Temperature dependence of DC conductivity analysis
The temperature-dependent electrical conductivity of the pure and doped solid electrolytes at a single frequency of 1 MHz is shown in Fig. 2. The conductivity of the samples follows the Arrhenius relation (Eq. 2.):
where σT is the total conductivity, σo is the pre-exponential factor, Ea is the activation energy of ionic motion, and k is the Boltzmann constant. Figure 2 displays the temperature dependence of the ionic conductivity (Arrhenius plots) of the bulk part for the samples. After the dissolution of KCl ingredient into the matrix of CuCl–CdCl2 heterogeneously, conductivity of the solid electrolytes was enhanced much greater than that of pure CuCdCl3. The ionic conductivity increased as the temperature increased, and the values of conductivity reached maximum for the sample with x = 0.3 composition at higher temperatures. This may be due to the fact that at high temperatures thermal disturbances through lattice vibrations take place. In such a high-temperature region, Cu+ readily excites and moves at short range, but the number of mutual collisions also increases resulting in a decrease of the Cu+ mobility. The σT of the sample with x = 0.3 composition displays high ionic conductivity of 3.94 × 10−5 Scm−1 at 320 °C. The activation energies of conduction obtained from the data between 293 and 593 K are plotted as a function of KCl composition in Fig. 3. As can be seen from the figure, those activation energies of the solid electrolytes slightly decreased upon increasing the amount of KCl contents up to x = 0.3, after that, they started increasing.
Figure 4 illustrates the electrical conductivity behaviour of the solid electrolytes at different concentrations and temperatures. As can be observed from the figure, conductivity of the samples increases with the amount of KCl additives that pass through a threshold value of x = 0.3. After that, decrease in the ionic conductivity values occurred with the further increase of KCl contents, by the blockage of the conduction path of the electrolyte. The principal reason behind the conductivity enhancement is due to the formation of conducting paths, which powerfully leads to richly conducting interface and can be explained sufficiently by space-charge theory [35].
Frequency dependence of AC conductivity analysis
The frequency-dependent ionic conductivity ranging from 10 kHz to 1 MHz of (CuCl)4−2x–(KCl)x–CdCl2 samples at 35 °C is shown in Fig. 5. The AC conductivity of solid electrolytes is firmly dependent on the structural disorder of samples and doping procedure [36, 37]. The highest conductivity for KCl-doped ionic system was obtained at higher frequency as shown in figure. This may be caused by small polaron hopping in the present samples [38]. The conductivity of KCl-doped solid electrolytes increases with respect to the applied frequency, which is due to excitation caused by active charges [39]. The conductivity value increases with the addition of KCl within the ionic system with respect to frequency at 35 °C of temperature which can be as a result of increase in hopping.
Conclusions
The ionic system CuCl–CdCl2-based solid electrolytes with different compositions of KCl were synthesised by solid-state reaction method. The structural characterization and thermal analysis of electrolytes were investigated on the basis of XRD and DSC data, respectively. AC impedance spectroscopy suggested that the ionic conductivity mainly resulted from grain effect. The electrolyte containing x = 0.3 composition shows the highest conductivity reaching 3.94 × 10−5 Scm−1 at 320 °C and lowest activation energy in the temperature range of 293–593 K. The experimental results show that the ionic conductivity of samples was enhanced by increasing the temperature, frequency, and KCl content that can be described by space-charge layer model. The DC conductivity and AC conductivity are almost the same at lower temperature because of rapid increment of the mobility charge carriers in electrolyte. Hence, the present study reveals that the change in conductivity value depends on concentration of doped ingredient as well as on various parameters in the system. Therefore, the result obtained from various characterizations implemented for the present solid electrolytes proposes it for the progress of different electrochemical applications.
References
Diptikanta, S., Tayur, N.G.R.: Structure, ionic conduction and dielectric relaxation in a novel fast ion conductor, Na2Cd(SO4)2. Chem. Mater. 19, 347–349 (2007)
Park, S.H., Praise, J.B., Gies, H., Liu, H., Grey, C.P., Toby, H.B.: A new porous lithosilicate with a high ionic conductivity and ion-exchange capacity. J. Am. Chem. Soc. 112, 11023–11024 (2000)
Bruce, P.G.: Solid State Electrochemistry. Cambridge University Press, Cambridge (1997)
Chandra, S.: Superionic Solids: Principles and Applications. North-Holland Publishing, Amsterdam (1981)
Takahashi, T. (ed.): High Conductivity Solid Ionic Conductors: Recent Trends and Applications. World Scientific, Singapore (1989)
Kendall, K.R., Navas, C., Thomas, J.K., Zur Loye, H.C.: Recent developments in oxide ion conductors: aurivillius phases. Chem. Mater. 8, 642–649 (1996)
Boivin, J.C., Mairesse, G.: Recent material developments in fast oxide ion conductors. Chem. Mater. 10, 2870–2888 (1998)
Goodenough, J.B.: Ceramic technology: oxide-ion conductors by design. Nature 404, 821–823 (2000)
Li, Z.: Impedance analysis and protonic conduction mechanism in RbH2PO4/SiO2 composite systems. Electrochim. Acta 55, 7298–7304 (2010)
Yamada, H., Moriguchi, I., Kudo, T.: Nano-structured Li-ionic conductive composite solid electrolyte synthesized by using mesoporous SiO2. Solid State Ion 176, 945–953 (2005)
Jain, A., Saha, S., Gopalan, P., Kulkarni, A.: Na2SO4–Al2O3composite electrolytes: some interesting observations. Mater. Res. Bull. 35, 2337–2342 (2000)
Kumar, A., Shahi, K.: Composition and particle size effects on ionic conduction in KCl–Al2O3 composite solid electrolytes. J. Phys. Chem. Solids 56, 215–222 (1995)
Urarov, N.F., Vangk, P., Yuzyuk, Y., Zeleznq, V., Studnicka, V., Bokhonov, B.B., Dulepov, V.E., Petzelt, J.: Properties of rubidium nitrate in ion-conducting RbNO3–Al203 nanocomposites. Solid State Ion 90, 201–207 (1996)
Uvarov, N.F., Brezhneva, L.I., Hairetdinov, E.F.: Effect of nanocrystalline alumina on ionic conductivity and phase transition in CsCl. Solid State Ion 136, 1273–1277 (2000)
Knauth, P., Auer, G.: Influence of the surface composition of TiO2 on the electrical conductivity of CuBr–TiO2 composites. Solid State Ion 147, 115–121 (2002)
Fujishiro, F., Mochizuk, S.: The interfacial effect on ionic conduction of AgI–anatase TiO2 composites. Solid State Ion 180, 497–500 (2009)
Sultana, S., Rafiuddin, R.: Electrical conductivity in TlI–TiO2 composite solid electrolyte. Phys. B 404, 36–40 (2009)
Liu, J., Du, R., Shi, R., Wang, H.: Facile modified synthesis and intermediate temperature electrical properties of Sn0.95Al0.05P2O7/KSn2(PO4)3 composite electrolyte. Ceram. Int. 44, 5179–5183 (2018)
Sun, L., Miao, H., Wang, H.: SrCe1−xYbxO3−α-(Na/K)Cl composite electrolytes for intermediate temperature solid oxide fuel cells. Solid State Ion 311, 41–45 (2017)
Beg, S., Salami, N.S.: Study on the electrical properties of Co–Ti double substituted Bi4V2O11. J. Alloys Compd. 586, 302–307 (2014)
Beg, S., Hafeez, S., Al-Areqi, A.S.: Study of phase stabilization and oxide–ion conductivity in BICUMGVOX solid electrolyte. Solid State Ion 261, 125–130 (2014)
Patro, L.N., Hariharan, K.: Ionic transport studies in Sn(1−x)KxF(2−x) type solid electrolytes. Mater. Res. Bull. 47, 2492–2497 (2012)
Lacorre, P., Goutenoire, F., Bohnke, O., Retoux, R., Lalignant, Y.: Designing fast oxide-ion conductors based on La2Mo2O9. Nature 404, 856–858 (2000)
Owens, B.B., Argue, G.R.: High-conductivity solid electrolytes: MAg4I5. Science 157, 308–310 (1967)
Wei, X., Shriver, D.F.: High ionic conductivity in some lithium halide systems. Chem. Mater. 12, 2528–2529 (2000)
Matsui, T., Wagner, J.B.: High conductivity cuprous halide-metal halide systems. J. Electrochem. Soc. 124, 937–944 (1977)
Matsui, T., Wagner, J.B.: Investigations on a high conductivity solid electrolyte system, RbCl + CuCl. J. Electrochem. Soc. 124, 941–944 (1977)
Kano, R., Takeda, Y., Masuyama, Y., Yamamoto, O., Takahashi, T.: Phase diagram and high copper ion conductivity of the copper(1) chloride-rubidium chloride system. Solid State Ion 11, 221–226 (1983)
Titilayo, A.K.: Structure and ionic conductivity of CuCdCl3. Solid State Ion 25, 105–108 (1987)
Chebotin, V.N.: Fizicheskaya Khimiya tverdogo tela (The Physical Chemistry of Solids). Khimiya, Moscow (1982)
Chebotin, V.N., Perfil’ev, M.N.: Elektrokhimiya tverdykh elektrolitov (The Electrochemistry of Solid Electrolytes). Khimiya, Moscow (1978)
Lucas, F.O., et al.: Structural, optical and electrical properties of Co-evaporated CuCl/KCl films. Phys. Status Solidi C 6(S1), S114–S118 (2009). https://doi.org/10.1002/pssc200881310
Dygas, J.R., Malys, M., Krok, F., Wrobel, W., Kozanecka, A., Abrahams, I.: Polycrystalline BIMGVOX.13 studied by impedance spectroscopy. Solid State Ion 176, 2085–2093 (2005)
Iqbal, M.Z., Rafiuddin, R.: Preparation, characterization, electrical conductivity and dielectric studies of Na2SO4 and V2O5 composite solid electrolytes. Measurement 81, 102–112 (2016)
Ponomareva, V.G., Lavrova, G.V.: The investigation of disordered phases in nanocomposite proton electrolytes based on MeHSO4 (Me = Rb, Cs, K). Solid State Ion 145, 197–204 (2001)
Panday, K., Dwivedi, M.M., Singh, M., Agarwal, S.L.: Studies of dielectric relaxation and a.c. conductivity in [(100 − x)PEO + xNH4SCN]: Al-Zn ferrite nano composite polymer electrolyte. J. Polym. Res. 17, 127–132 (2010)
Haldar, I., Biswas, M., Nayak, A.: Preparation and evaluation of microstructure, dielectric and conductivity (ac/dc) characteristics of a polyaniline/poly N-vinyl carbazole/Fe3O4 nanocomposite. J. Polym. Res. 19, 9951–9955 (2012)
Gabal, M.A., Al Angari, Y.M.: Effect of diamagnetic substitution on the structural, magnetic and electrical properties of NiFe2O4. Mater. Chem. Phys. 115, 578–584 (2009)
El Ghanem, H.M., Jawad, S.A., Al-Saleh, M.H., Hussain, Y.A., Salah, W.: Effect of dc-bias on the dielectric behavior of CNT/ABS nanocomposites. Phys. B 418, 41–46 (2013)
Acknowledgements
The authors are thankful to the Chairman, Department of Chemistry, AMU Aligarh, for providing all necessary facilities which were required for the research work and UGC, New Delhi, India, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wani, S.I., Rafiuddin, R. Impedance spectroscopy and conductivity studies of KCl-doped solid electrolyte. J Theor Appl Phys 12, 141–146 (2018). https://doi.org/10.1007/s40094-018-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-018-0294-z