Abstract
Purpose
In this study, vermicomposted tannery wastes were applied in the organic cultivation of sweet pepper in a greenhouse. The effects of this organic matter addition on plant development and on the distributions of Cr(III) and Cr(VI) in plant tissues and organs were assessed.
Methods
In a greenhouse, organic sweet peppers were cultivated adding vermicomposted tannery to the substrate (sample VRC) and the results were compared with the samples control (dYL; only soil) and reference (NPK; adding mineral fertilizer). The growth, nutritive value and fruit production were assessed to evaluate the plant development in different types of substrate. Besides evaluating the biostimulant effect of vermicomposts, the distributions of Cr in plant tissues were also studied.
Results
Up to three harvested sweet peppers were obtained per plant, compared with only one fruit for the reference treatment with NPK addition. Based on the Cr dynamics in the plants, the highest concentration was found in the fruits, varied as follows: fruits > stem and stalks > leaves = root; however, the Cr concentration in the fruits was statistically the same across all treatments. Additionally, only Cr(III) was detected and quantified in the fruits.
Conclusion
The addition of vermicompost was biostimulating to plants and positively influenced their development. Based on the Cr dynamics in the plants, since the Cr concentration was the same across all treatments, it demonstrated that the addition of tannery residues to the vermicompost did not negatively influence the health benefits or food security of the produced fruits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Brazil, annually an amount of ~ 44.7 thousand metric tons of leather is manufactured. To hold this high production, approximately 450 tanneries plants, located all over the country, make Brazil the fourth leading producer in the world, behind China (209.8), India (52.7), and Italy (51.7 thousand metric tons) (CETESB 2005).
Environmental toxins and demand for safe disposal of industrial wastes are currently the topics of concern. The leather industry is associated with the generation of huge amounts of solid wastes, and waste disposal is a serious problem. Untreated solid waste and wastewater generated by the tanning industry can cause serious environmental damage, particularly to surface water and groundwater (Belay 2010; Vig et al. 2011; Dixit et al. 2015; Scaglia et al. 2016).
Usually, Cr is introduced mainly in the form of Cr2(SO4)3 [chromium (III) sulfate] aiming to stabilize the leather by cross linking the collagen fibbers. As final product, the tanned skin by adding Cr salts shows a better texture and quality, resulting in manufactured products with a higher market value. Besides Cr, other contaminants are added in the tanning process, e.g., sodium and ammonium sulfate, thiobenzothiazole, kerosene, etc., but in small concentration. To avoid such pollution, specific chemical, physical and biological treatments have been adopted to reduce waste toxicity and enhance tannery waste use/recycling systems (Aira et al. 2008; Yang et al. 2014; Scaglia et al. 2016; Makkar et al. 2017).
In this context, vermicomposting is an effective technology that can reduce the toxic effects of tannery wastes, based on earthworm activity of Eisenia fetida L., which also improves nutrient availability of OM. Earthworms transform organic residues both physically and chemically by mixing and grinding the waste mass, whereas microbes in the guts of worms are responsible for the biochemical transformation of OM (Aira et al. 2008; Yang et al. 2014; Scaglia et al. 2016; Makkar et al. 2017). Moreover, earthworms can accumulate heavy metals in their chloragogen cells, resulting in detoxification of polluted biomass (Huges 1980; Ricci 1996; Nunes et al. 2016; Karimi et al. 2017).
Several forms of industrial waste (i.e., paper mill sludge, sugar industry waste and textile waste) have been successfully vermicomposted to obtain useful and safe organic inputs (Ravindran et al. 2008; Vig et al. 2011). In addition, vermicompost applications increase the yield and growth of several crops, mainly because these organic inputs are rich in plant-available nutrients, such as phosphates, nitrates, and exchangeable soluble potassium and calcium (Arancon et al. 2006; Bachman and Metzger 2008; Gopinath et al. 2009). The use of organic amendments for improving soil productivity and health has been reported previously, usually to provide a source of nutrients in organically managed systems, particularly in organic agriculture practices (Ramesh et al. 2006; Gopinath et al. 2009; Singh et al. 2011; Karimi et al. 2017).
Sweet pepper (Capsicum annuum L.) is a highly consumed vegetable that is better adjusted to and, therefore, most frequently produced under greenhouse conditions (Pereira 1990; Scivittaro et al. 1999; Silva et al. 2002; Herman et al. 2008). Greenhouse cultivation of sweet pepper demands the intensive use of agri-inputs and labor; moreover, this intense activity is part of a dynamic market in which seasonal prices fluctuate, demanding rigorous planning by the producer for both crop production and the use of technology to decrease production risks and increase profits (Pereira 1990; Silva et al. 2001; Abul-Soud et al. 2014).
Vermicompost fertilization is well known for its effects on plant nourishment, which result in reduced requirements for (or the elimination of) chemical pesticides and inorganic fertilizers. Additionally, an understanding of the effects of OM addition on sweet pepper plants at each stage of development can provide information to assist with fertilizing programs for crops, even in greenhouses (Bachman and Metzger 2008; Llaven et al. 2008; Gopinath et al. 2009; Abul-Soud et al. 2014).
Therefore, the aim of this work was to determine the effects of application of vermicomposted tannery wastes on organic sweet pepper cultivation in the greenhouse. In particular, the effects of this organic matter addition on plant development, i.e., growth, nutritive value and fruit production, were assessed. Additionally, the distribution of chromium, a heavy metal in tannery wastes, was determined in pepper plant tissues and organs.
Materials and methods
Vermicompost production
This study was conducted using vermicomposted tannery wastes in the form of both sludge and tanned chips mixed with cattle manure and sawdust. The vermicomposters were established in 25 L plastic barrels containing different proportions of fresh wastes (based on dry volume). The proportion of the residues was determined by a combination of their C:N ratios. Designed in triplicate, each barrel received 20 L of the mixture of wastes as follows, based on fresh volume: (1) sludge mix (VRC—S): 14.5 L of cattle manure, 2.5 L of sawdust, and 3 L of sludge; (2) tanned chips mix (VRC—C): 14 L of cattle manure, 2 L of sawdust, and 4 L of tanned chips; and (3) sludge + tanned chips mix (VRC—SC): 14 L of cattle manure, 2 L of sawdust, 1.5 L of sludge, and 2.5 L of tanned chips. For comparison, a vermicomposter with no leather wastes was also prepared (standard treatment, VRC—STD) by mixing fresh-volume 17 L of cattle manure and 3 L of sawdust.
Previously analyzed, the raw materials presented as main characteristics: (1) sludge (S): pH 7.83, OM 36.32%, TOC 17.20%, N 4.63%; (2) tanned chips (C): pH 3.31, OM 84.96%, TOC 48.26%, N 13.30%; (3) cattle manure: pH 8.88, OM 63.02%, TOC 34.30%, N 2.02%; and (4) sawdust pH 4.84, OM 89.11%, TOC 52.69%, N 0.43%.
After the mixtures were prepared, the material stabilized for 1 week; then, all of the contents were turned manually once per week. For vermicompost production, 500 newly born earthworms (Eisenia fetida L.) were added to each vermicomposter. The experiment was conducted during the months of April–August 2014 (135 days) in the Laboratory of Environmental Chemistry (LQA), Institute of Chemistry of São Carlos (IQSC), University of São Paulo (USP), Brazil (Nunes et al. 2016).
To characterize the vermicomposts, 0.5 L samples were collected from each vermicomposter. Then, a single combined sample was prepared from the triplicate samples. The material was air-dried, ground and sieved to 0.5 mm. In triplicate, the OM (NEN 5754 1994), total organic carbon (TOC) (ISO 10694 1995), pH (ISO 10390 1994), cation exchange capacity (CEC) (ISO 11260 1994), humic acid content (HA) (Swift 1996), macro and micronutrients (EMBRAPA 2017), and C:N ratio (using a Perkin Elmer 2400; North Billerica, MA, USA) were determined in the vermicomposts.
Sweet pepper organic cultivation
Samples of a dystrophic yellow latosol (dYL) were collected on Campus II of the University of São Paulo (21°59′51″S, 47°56′20″W). Using a tractor, 1.0 ton of soil was drilled at a 40.0 cm depth in the arable fraction. Thereafter, the material was sieved to 2.0 mm and air-dried. Soil collection and preparation were conducted in February 2014, which had an average temperature of 24 °C and precipitation of 198 mm.
To characterize the dYL soil, the following attributes were determined: pH, organic matter (OM), total organic carbon (TOC), cation exchange capacity (CEC), base saturation (BS), macro- and micronutrients, particle-size distribution, and soil texture.
Sweet pepper seedlings (biologically certified) were purchased from SouAgro—Sociedade Agropecuária (São Carlos, SP, Brazil). Each seedling was ~ 3.0 cm in height and ~ 30 days from planting. Seedlings were grown on a commercial substrate of coconut fibre and pine bark (Bioplant®, Nova Ponte, MG, Brazil) and were watered manually daily.
When seedlings reached 5.0 cm in height and had at least five permanent leaves, in addition to a healthy appearance, the seedlings were transplanted into 0.5 L plastic pots using the same commercial substrate. At 10 cm in height, the plants were transplanted to 25.0 L vessels for the beginning of the experiment.
Each seedling was grown in a 25.0 L vessel containing 20.0 L of substrate. The cultivation was monitored daily, and to prevent pests, an aqueous extract of orange peel was sprayed on the leaves, a natural repellent that insects avoid. The invasive plants were eliminated manually. Using a watering can (manual watering), 1.0 L of water was added daily to each plant. The volume of 1.0 L was determined from the wilting point, obtained by preliminary tests.
For the organization of the experiment, the vessels were distributed randomly on benches, with 42 vessels distributed on two benches, constructed from two boards on three tripods, with a size of 6.0 × 0.6 m. In total, 24 vessels were arranged in a 2 × 12 system (column × row) and the other 14 vessels in a 2 × 7 system (column × row). Each column corresponded to one board. The distance between the vessels was 10.0 cm and positions were changed weekly at random. Positions were modified to minimize possible external influences, e.g., shade and wind.
Agronomic assays were carried out in triplicate. According to the different types of substrates, the following treatments were studied: SP—dYL: Soil without previous management (control treatment); SP—NPK: Soil and 3.0% (V/V) of mineral NPK fertilizer at 12:5:14 (reference treatment); and SP—VRC: Soil and vermicomposts at concentrations of 1.5, 3.0 and 6.0% (V/V) (Table 1).
Monitoring of sweet pepper cultivation
Sweet pepper growth was monitored biweekly using a metric scale. The height (stalk size) of the plants was measured from the base (substrate) to the culm apex (highest point of the stem). The number of fixed leaves (below the apex) was counted and recorded every 2 weeks for each plant. In the first month, the count was weekly.
The width (w) and length (h) of the five largest leaves of each plant were measured every 15 days. From these data, the average leaf area (A) was estimated (Eq. 1) (Lorenzeni et al. 2015).
Four periods related to flowering and the formation of green and mature fruits were assessed with daily monitoring. The average flowering time was based on initiation of flowering. Fruit formation was measured from shortly after petal fall. Formation of stable green fruits was determined by size and appearance (intense green). The average maturation time was determined by formation of stable, full-red (mature) fruits.
The average flowering time was calculated by the difference in days between the appearance of the flowering and the falling of petals. The difference in days between the complete formation of the green fruit and the intense red coloration of the fruit was used to determine the mean maturation time.
The size, circumference and weight were measured of fruits that were well shaped and of appropriate color. Then, the fruits were dehydrated at 40 °C for future determinations. Fruits containing anomalies or that were malformed were counted but not measured and weighed.
The chromium concentration was quantified using an Atomic Absorption Perkin Elmer, model PINAACLE 900T (Waltham, MA, USA). Cr(III) and Cr(VI) were extracted in deionized water using mechanic agitation at 60 rpm for 30 min, followed by vacuum filtration.
At post-harvest, because of the addition of Cr-rich waste, Cr was quantified in all the organs and tissues of the plants to determine possible heavy-metal accumulation in sweet pepper. After being cropped, the plants were separated in roots, stem and stalks, leaves, and fruits. All the analyzed replicates were mixed to form a single sample, which was analyzed in triplicate.
Statistical analyses
Following one-way bootstrap ANOVA, the differences among means were evaluated using Duncan’s multiple range tests at p < 0.05. The IBM SPSS statistical software package v. 20 was used for data analyses.
Results and discussion
Substrate characterization
To determine the nutritive potential of the vermicomposts, macro- and micronutrient contents and the chemical characteristics were analyzed, as shown in Table 2. When compared with the control vermicompost (VRC—STD), the attributes of vermicomposts produced from tannery residues (VRC—S, VRC—C and VRC—SC) related to agricultural fertility, e.g., OM, TOC, and CEC, among others, were not significantly different (ANOVA bootstrap, p > 0.05).
For macronutrient contents, the VRC—STD had higher concentrations of P (0.70%), K (3.19%) and Mg (0.78%) than tannery-based vermicomposts, whereas VRC—S had higher values of Ca (2.11%) and S (0.49%). The lowest N value (1.32%) was in VRC—C, but the concentrations of other macronutrients were not affected. The VRC—SC had the lowest values of all vermicomposts for P (0.59%), K (0.88%), Ca (0.52%) and S (0.36%). Although the determined values were different, the concentrations did not exceed 1.00% (Table 2).
When micronutrient contents were compared, the differences were extreme (Table 2). Except for Fe, the concentrations of micronutrients in VRC—C always greatly exceeded those in the others: B (577.67 mg kg−1), Cu (602.28 mg kg−1), Mn (570.22 mg kg−1) and Zn (499.17 mg kg−1), i.e., values approximately fivefold higher than those determined in VRC—SC. However, the values observed in these two vermicompost types were up to 10,000-fold greater than concentrations determined in the VRC—STD and VRC—S. Therefore, micronutrient quantities were found from high to low as follows: VRC—C > VRC—SC > VRC—S > VRC—STD.
For the content of Cr, as expected, VRC—STD had values of Cr(III) and Cr(VI) below the detectable level (LOQ, 79.80 μg kg−1). In the vermicomposts containing tannery residues, the concentrations of Cr(III) were VRC—C at 7.47 mg kg−1, VRC—S at 4.33 mg kg−1 and VRC—SC at 1.09 mg kg−1, with all values significantly different (ANOVA bootstrap, p < 0.05). For Cr(VI), this form was not detected in any vermicompost (Table 2).
Sweet pepper cultivation
The attributes of the latosol were typical of a dYL soil (EMBRAPA 2018), i.e., acidic with a pH of 5.74, poor OM content of 1.52%, TOC of 0.25%, CEC of 64.91 cmolc kg−1, and small base saturation of BS 27.99%. For soil particle size distribution, the texture of the dYL was classified as sandy clay loam, with 31.00% clay, 11.00% silt and 58.00% sand (Table 3).
For nutrients, the dYL had low levels of essential elements for plants (Table 3). Therefore, the dYL did not satisfy the nutritional requirements for cultivation for agricultural production, with low levels of the exchangeable bases: K (0.33 cmolc kg−1), Ca (13.03 cmolc kg−1) and Mg (4.81 cmolc kg−1). For micronutrients, the concentrations from high to low were Mn (337.21 mg kg−1), Zn (191.26 mg kg−1), Cu (179.03 mg kg−1) and Fe (59.19 mg kg−1). Levels of Na and some other micronutrients were below the detectable level (LOQ) (Table 3).
Poor in OM and with low CEC and nutrients, the dYL was ideally suited to evaluate the nutritive potential of the vermicomposts. A soil rich in OM could confound interpretation of the results following vermicompost application because the effects of soil OM and OM added from the vermicompost on plant nutrition cannot be discerned individually. Additionally, incorporating OM into soil leads to improved CEC and BS, in addition to increasing TOC levels, which are fundamental attributes for soil quality in agricultural systems (Ndegwa et al. 2000; Canellas and Santos 2005; Tobiašová 2011).
Criteria were established for the transfer of seedlings from the trays to the plastic pots and then to the cultivation vessels: 5- and 10-cm height, respectively. Approximately, 7 and 30 days passed for each transplant, respectively.
In general, plants from the SP—dYL and SP—NPK treatments had distinctly less development compared with those from SP—VRC. Plants grown on substrates containing vermicomposts showed more growth and more fruits were produced, with better appearance and greater weight and dimensions. Generally, as the amount of vermicompost increased, the yield (number of fruits) and plant health (visual analysis) increased.
The development of sweet pepper plants in SP—dYL and SP—NPK (control and reference treatments) was similar; however, fruits were only produced on plants in the SP—NPK treatment. Although vermicomposts with different compositions have been examined in previous studies, similarly satisfactory results have been reported (Bachman and Metzger 2008; Kaur et al. 2010; Vig et al. 2011; Kenyangi and Blok 2013; Nunes et al. 2016). Data related to the development of sweet pepper plants (e.g., growth, nutritive value, and fruit production, among others) obtained in this study are discussed below.
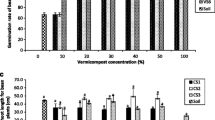
Plant growth in treatments SP—dYL and SP—NPK was not significantly different at 12.7 and 19.0 cm, respectively (ANOVA bootstrap, p < 0.05; Table 4). Therefore, the addition of NPK fertilizer did not significantly affect plant growth. Plant growth in the control and reference treatments was significantly different from all treatments in the SP—VRC group at all studied concentrations (ANOVA bootstrap, p < 0.05).
Plants cultivated with the control vermicompost (VRC—STD) added at 1.5%, 3.0% and 6.0% did not show significant differences in growth (ANOVA bootstrap, p < 0.05), after 120 days of the experiment. The difference in plant growth was ~ 6–28%, with mean growth between 32.0 and 41.0 cm (Table 4).
Analyzing plant growth under application of the vermicompost S, the mean growth of plants in treatments SP—1.5%—S and SP—3.0%—S was not significantly different at 24.7 and 30.3 cm, respectively. However, both levels of growth were significantly different compared with that of plants in the treatment SP—6.0%—S, with growth of 43.7 cm (ANOVA bootstrap, p < 0.05; Table 4).
When vermicompost C was applied, mean plant growth in treatments SP—3.0%—C and SP—6.0%—C was 45.0 and 49.7 cm, respectively, which were not significantly different. Plants in the treatment SP—1.5%—C grew 32.0 cm, which was significantly different from growth in the other levels of vermicompost C (ANOVA bootstrap, p < 0.05). At all concentrations, plant growth in the SP—SC treatments was not significantly different compared with that in the SP—C treatments (Table 4).
In general, the growth of all plants that received 1.5% vermicompost was not significantly different (ANOVA bootstrap, p > 0.05). Among all treatments, plants with the highest growth were in SP—6.0%—C and SP—6.0%—SC, both at ~ 49 cm. In samples from the group SP—VRC, plant growth was proportional to vermicompost concentration, with growth at 1.5% < 3.0% < 6.0% (Table 4).
Data related to the leaves (e.g., number, area, and nutritive value) were important to monitor and to qualify in the development of sweet pepper plants in different types of substrate. From the beginning of the experiment (~ 15 days after planting), the uniqueness in the dimensions of leaves could be observed. After 45 days, whereas the size of leaves from the SP—dYL and SP—NPK treatments remained with the same, leaves in the SP—6.0%—SC treatments had dimensions that were larger than a hand. For the leaf area (A), after 60 days, the area of leaves in SP—dYL was significantly smaller than that in the other treatments (8.33 cm2). In SP—NPK and SP—1.5%—VRC treatments, the areas of leaves were not significantly different, with values varying between 23.82 and 31.27 cm2 (ANOVA bootstrap, p < 0.05; Table 4).
In general, the nutrient contents in the leaves varied randomly, without tending to the expected. As the sweet pepper continued to develop, the concentrations of nutrients decreased as expected, primarily because of ion dilution in the tissues and organs of the plants (Tables 5 and 6) (Malavolta 1980; Malavolta et al. 1997); however, exceptions were observed.
Nutrients directly influenced plant development, as demonstrated by sweet pepper growth and production. Additionally, visual assessment of the plants, based primarily on the color, shape and texture of leaves, provided important information on the nutritional statuses of the plants (Malavolta 1980; Epstein 1997; Faquin 2005; Epstein and Bloom 2006; Fontes 2006).
Concerning the appearance of leaves, a yellowing was observed in plants of the SP—dYL treatment after 5 days of cultivation that persisted for the entire experiment, with the same occurring after 45 days for plants of the group SP—1.5%—VRC. At concentrations of 3.0% and 6.0% vermicompost, the yellowing occurred at 75 and 90 days of cultivation, respectively. In the SP—NPK treatment, no change was observed in leaf color throughout the experiment. Although the application of mineral fertilizer did not contribute significantly to plant development, nutrition was guaranteed at all plant developmental stages.
For plants that received the vermicompost control, leaf area in the SP—1.5%—STD treatment (27.24 cm2) was significantly different from that of the others (43.12 cm2 in SP—3.0%—STD and 60.73 cm2 in SP—6.0%—STD), with the difference between the two higher levels not significant. Leaf area of plants from SP—C and SP—SC treatments was not significantly different and their leaves had the largest dimensions and therefore areas (ANOVA bootstrap, p < 0.05).
For macronutrients (Table 5), in a comparison between the control and reference treatments (SP—dYL and SP—NPK, respectively), the concentrations in plants from SP—NPK were higher than those in the control, as expected. However, concentrations of K (5.71%) and S (0.79%) were the exceptions and were below those of plants in SP—dYL. Furthermore, the addition of N, P and K from the mineral fertilizer did not guarantee the highest concentrations of these nutrients among treatments, except for N (2.29%). The highest value of P was determined in SP—6.0%—S (0.67%) and that of K in SP—1.5% —STD (6.63%). The lowest concentrations of macronutrients were in SP—C and SP—SC treatments, with the lowest values of P (0.18% in SP—3.0%—SC), K (2.16% in SP—6.0%—SC) and S (0.71% in SP—1.5%—SC), most likely due to ion dilution in the plant tissues (Malavolta 1980; Malavolta et al. 1997).
Concerning the micronutrients (Table 6), the highest concentrations were in plants from the SP—6.0%—STD treatment, with high levels of B (164.12 mg kg−1), Zn (145.84 mg kg−1) and Fe (1316.88 mg kg−1); and in the plants from SP—SC treatments, with high levels of Cu (29.64 mg kg−1 in SP—3.0%—SC) and Mn (492.57 mg kg−1 in SP—3.0%—SC). With the exceptions of Zn and Fe, the lowest concentrations of micronutrients were in plants from the SP—C treatments: B (50.64 mg kg−1) and Cu (0.04 mg kg−1) in SP—6.0%—C; and Mn (100.14 mg kg−1) in SP—3.0%—C.
Consistent with our findings, many studies have demonstrated the effects of nutrients on growth, development and fruit production in sweet pepper (Malavolta 1980; Marti and Mills 1991; Aguilera-Gomez et al. 1999; Riga and Anza 2003; Faquin 2005; Albuquerque et al. 2011).
Marti and Mills (1991) studied the effects of Ca uptake by roots on plant growth and development, in addition to its correlation with the N concentration in the substrate. Albuquerque et al. (2011) studied the effect of the soil K concentration on the production of red sweet pepper, in addition to that on biometric fruit data. Riga and Anza (2003) evaluated the effects of Mg deficiency on the physiology of pepper plants and found that it affected plant growth and development. Moreover, Aguilera-Gomez et al. (1999), among other studies, examined the effects of P uptake on the development of sweet pepper plants. Overall, vermicomposts clearly sufficiently nourished the sweet pepper plants to promote their growth, development and fruit production.
Fruit production (red sweet pepper, at the mature stage), in addition to fruit characteristics (dimensions, weight and appearance), are important attributes for evaluating the effects of vermicompost addition on sweet pepper cultivation. In general, the addition of vermicompost was a biostimulant to the plants in this study, increasing the number and quality of cropped fruits (Table 7).
Regarding production, plants subjected to the control treatment (SP—dYL) did not produce any fruit, whereas those exposed to the reference treatment (SP—NPK) produced only one harvested fruit per plant. The optimum results were obtained for the plants exposed to the SP—3.0%—SC and SP—6.0%—SC treatments, which produced three fruits per plant. In addition, the plants subjected to the SP—6.0%—S, SP—3.0%—C, and SP—6.0%—C treatments produced two fruits. For the other treatments, the plants only produced one fruit (Table 7).
Some plants also produced red sweet peppers that did not meet esthetic standards, with irregular shape and coloration, resulting in no commercial value. The worst results were observed for plants in the treatments of group SP—STD because for each two well-formed fruits, one fruit was an anomaly (50% of production). Some treatments showed a significantly higher proportion of well-formed fruits, including in SP—6.0%—SC (5:1 ratio), with only two of ten total fruits showing anomalies, and in SP—3.0%—SC (ratio 8:0), SP—6.0%—C (ratio 6:0), and SP—3.0%—C (ratio 4:1) (ANOVA bootstrap, p < 0.05). Plants exposed to some of the treatments showed only well-formed fruits, i.e., SP—6.0%—C, SP—3.0%—SC and the reference treatment, SP—NPK (Table 7).
For fruit formation, less time was required for formation and maturation of red sweet peppers as the vermicompost concentration increased, except for the plants in the SP—C treatments, which all had maturation times between 92 and 110 days that were not significantly different (ANOVA bootstrap, p < 0.05). Additionally, plants in all the treatments with 6.0% vermicompost, some treatments with 3.0% (i.e., SP—3.0%—STD, SP—3.0%—C and SP—3.0%—SC) and the SP—NPK reference treatment showed maturation times that were not significantly different at 87–105 days (ANOVA bootstrap, p < 0.05, Table 7).
In general, fruits with shorter maturation times had higher weights, varying between 100.8 and 134.5 g, with no significant differences (ANOVA bootstrap, p < 0.05). Fruits obtained on plants from treatments with 1.5% vermicompost had fruits that weighed between 23.1 and 46.7 g, with values that were significantly different (ANOVA bootstrap, p < 0.05). The difference between fruit weights in the treatments SP—STD and SP—VRC was approximately 480%, indicating the biostimulant effect of vermicompost application on fruit production and attributes.
Concerning the dimensions of fruits, a relation between size and circumference could not be established because larger fruits could have a smaller circumference and vice versa. In general, plants that received 3.0% and 6.0% vermicompost produced fruits with the largest dimensions, varying between 10.5 and 12.5 cm in size and 17.0 and 23.0 cm in circumference, with no significant differences (ANOVA bootstrap, p < 0.05). In the treatments that received 1.5% vermicompost, the fruit size and circumference ranged between 6.0 and 8.0 cm and 11.2 and 13.3 cm, respectively (Table 7).
Ribeiro et al. (2000) studied the use of vermicomposts as an alternative for the organic production of sweet pepper in a protected environment. Additionally, the authors compared their results with experiments using a eutrophic red argisol (eRA) and under the application of NPK, imitating a conventional plantation. Although the structure and design of their study were similar to those of this study, the soil selected was different. An eRA (4.26% OM) could mask the results and influence of vermicompost on sweet pepper development because this soil had a certain nutritional potential, different from that of the dYL (EMBRAPA 2018). These findings are similar to those observed in our study. However, in that study, plants that received vermicompost had better results than those planted with NPK and in the control soil based on the following: (1) in organic cultivation (with vermicompost), the production was 16.0 t ha−1, whereas in the conventional planting, production was below 13.1 t ha−1; (2) the production in the control treatment (eRA) was below that obtained in organic cultivation but above that in conventional planting at 13.60 t ha−1; and (3) organic sweet peppers had an average weight of 72.9 g, compared with 67.8 g in the conventional and 65.5 g in eRA treatments.
In a comparison of studies, the fruit weight (84.6–134.5 g), for example, when 3.0% and 6.0% vermicompost were applied in our study, was notably higher than the 72.9 g found by Ribeiro et al. (2000). For fruit size, our organic sweet peppers also had larger dimensions, 9.3 cm in circumference, than the 9.0- and 8.9-cm circumferences found in the conventional cultivation and in eRA, respectively. Although our results were apparently superior, both studies indicated the efficacy and viability of the use of vermicompost in systems of organic agriculture and sweet pepper cultivation.
In general, because of the variety of soils in Brazil and the wide availability of agricultural wastes, scientific works are produced with different aims and goals, making comparisons among studies difficult. However, in all studies reported in the literature, specifically in the areas of environmental chemistry or agronomy and focused on organic agriculture, results and conclusions are consistent with those of this study; organic wastes prepared as inputs for organic agriculture can be applied in the planting of sweet pepper or other crops of economic and social interest.
Chromium
For the dynamics of Cr, the absorption of Cr(III) begins at the root, followed by transport through the tissues of the stem and stalks, reaching the leaves and fruit (Gropper et al. 2009; Thor et al. 2011; Hua et al. 2012). In this study, in some of these stages, Cr accumulation occurred (Table 8).
Initially, the root is expected to act as a type of filter that prevents assimilation of an excessive concentration of Cr by the plant, with accumulation in root tissues (Gropper et al. 2009; Thor et al. 2011; Hua et al. 2012). In the rhizosphere, the Cr(III) levels varied among treatments, although no trend was observed, with values ranging from 23.86 to 65.12 μg kg−1. However, for the treatments SP—dYL, SP—NPK, SP—STD, and SP—1.5%—S, Cr(III) was below the detectable level in roots, indicating metal absorption followed by transport, without accumulation in the root system.
In general, the Cr(III) concentrations varied as follows: fruits > stem and stalks > leaves = root, with exceptions (Table 8). The Cr(III) levels in fruits, stems and stalks were higher than those in the other tissues, most likely because of their abilities to accumulate metals in their cells and tissues. In the leaves, the levels of Cr(III) were not significantly different among the treatments, ~ 41 μg kg−1. In the stems and stalks, the levels of Cr(III) in the treatments SP—dYL, SP—NPK, SP—STD, and SP—1.5%—S were statistically similar, ranging between 60.71 and 64.50 μg kg−1; in the other treatments, levels of Cr(III) were also not significantly different, ranging from 82.19 to 92.44 μg kg−1 (ANOVA bootstrap, p < 0.05; Table 8).
Finally, the fruits are the organs that are of greatest concern because the food security of the consumer is ultimately affected (Gropper et al. 2009; Thor et al. 2011; Hua et al. 2012). In the sweet peppers, Cr(III) concentration varied between 145.06 and 165.20 μg kg−1. Fruits cropped in the treatments SP—NPK and SP—STD, at all concentrations, had levels of Cr(III) that were statistically similar. The Cr(III) levels determined in the treatments involving vermicomposted tannery residues were also statistically similar (SP—S, SP—C and SP—SC; ANOVA bootstrap, p < 0.05; Table 8). Additionally, tannery residues added in vermicomposting did not increase the Cr contents in fruit because the fruit concentrations of Cr(III) were statistically similar to those quantified in the fruits that received the vermicompost control (VRC—STD) at the same concentration (1.5%, 3.0% or 6.0% V/V).
When comparing the Cr(III) determined in the fruits in this study with other foods from different studies that are commonly reported as ‘Cr-rich’ or a ‘Cr-source’, at the level of chromium enrichment in our cropped red sweet peppers, the fruits would be available for human consumption (Table 9). For example, broccoli has 22.0 μg Cr 100 g−1, and brown bread and potatoes have 4.4 and 1.5 μg Cr 100 g−1, respectively. In red and green grapes, the Cr concentrations are 6.5 and 2.1 μg Cr 100 g−1, respectively. In our study, red sweet pepper showed, in general, concentrations ranging from 3.8 to 21.4 μg Cr 100 g−1, which are close to the concentrations present in other foods (Vique et al. 1997; Oliveira and Marchini 2008; NHI 2012).
Conclusions
The present study confirmed the expectation that the vermicomposted tannery wastes can be applied in the organic cultivation of sweet pepper. The vermicompost addition was biostimulating to the plants, influencing development positively, in addition to increasing fruit production. Although the fruits obtained in the SP—NPK (reference treatment) were statistically similar in characteristics to those cultivated with vermicomposted tannery wastes, the production in this treatment was limited to one fruit per plant, whereas in other treatments, up to three red sweet peppers were harvested per plant. Based on the results obtained in the agronomic assays, the suggested dosage to be applied in the organic cultivation of sweet pepper was the 6.0% of vermicompost. Also, mixing both forms of residues, sludge and tanned chips, it is possible to reach a best agronomic efficiency of the vermicompost, by increasing significantly the fruit production. When the levels of Cr were investigated in the plant tissues and organs, only Cr(III) was detected and quantified, as expected. The highest Cr content was found in the fruits; however, the Cr content was the same in fruits in all treatments, which demonstrated that the addition of tannery residues in the vermicompost did not negatively influence the quality, health or food security of the produced sweet peppers.
References
Abul-Soud MA, Emam MSA, Abdrabbo MAA et al (2014) Sustainable urban horticulture of sweet pepper via vermicomposting in summer. J Adv Agric 3:110–122. https://doi.org/10.13140/RG.2.1.1491.9201
Aguilera-Gomez L, Davies FT Jr, Olalde-Portugal V et al (1999) Influence of phosphorus and endomycorrhiza (Glomus intraradices) on gas exchange and plant growth of chile ancho pepper (Capsicum annuum L. cv. San Luis). Photosynthetica 36:441–449. https://doi.org/10.1023/A:1007032320951
Aira M, Sampedro L, Monroy F, Domínguez J (2008) Detritivorous earthworms directly modify the structure, thus altering the functioning of a microdecomposer food web. Soil Biol Biochem 40:2511–2516. https://doi.org/10.1016/j.soilbio.2008.06.010
Albuquerque FS, Silva EFF, Albuquerque Filho JAC, Nunes MFFN (2011) Growth and yield of sweet pepper fertigated under different irrigation depths and potassium doses. Rev Bras Eng Agrícola e Ambient 15:686–694. https://doi.org/10.1590/S1415-43662011000700006
Arancon NQ, Edwards CA, Lee S, Byrne R (2006) Effects of humic acids from vermicomposts on plant growth. Eur J Soil Biol 42:S65–S69. https://doi.org/10.1016/j.ejsobi.2006.06.004
Bachman GR, Metzger JD (2008) Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresour Technol 99:3155–3161. https://doi.org/10.1016/j.biortech.2007.05.069
Belay AA (2010) Impacts of chromium from tannery effluent and evaluation of alternative treatment options. J Environ Prot 01:53–58. https://doi.org/10.4236/jep.2010.11007
Canellas LP, Santos GA (2005) Humosfera. CCTA/UENF, Campos dos Goytacazes
CETESB (2005) Curtumes. Companhia Ambiental do Estado de São Paulo, São Paulo
Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87:39–49. https://doi.org/10.1016/j.jclepro.2014.10.017
EMBRAPA (2017) Manual de métodos de análise de solo. EMBRAPA Solos, Rio de Janeiro
EMBRAPA (2018) Sistema brasileiro de classificação de solos. EMBRAPA Solos, Rio de Janeiro
Epstein E (1997) The science of composting. Technomic Publishing Company, Lancaster
Epstein E, Bloom AJ (2006) Nutrição mineral de plantas: princípios e perspectivas. Editora Planta, Londrina
Faquin V (2005) Nutrição mineral de plantas. Editora UFLA, Lavras
Fontes PCR (2006) Diagnóstico do estado nutricional das plantas. UFV, Viçosa
Gopinath KA, Saha S, Mina BL et al (2009) Bell pepper yield and soil properties during conversion from conventional to organic production in Indian Himalayas. Sci Hortic (Amst) 122:339–345. https://doi.org/10.1016/j.scienta.2009.05.016
Gropper SS, Smith JL, Groff J (2009) Advanced nutrition and human metabolism. Cengage Learning, Boston
Herman MAB, Nault BA, Smart CD (2008) Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot 27:996–1002. https://doi.org/10.1016/j.cropro.2007.12.004
Hua Y, Clark S, Ren J, Sreejayan N (2012) Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem 23:313–319. https://doi.org/10.1016/j.jnutbio.2011.11.001
Huges HBN (1980) The biology of polluted waters. University Press, Liverpool
ISO 10390 (1994) Soil quality: determination of pH. International Organization for Standardization, Genebra
ISO 10694 (1995) Soil quality: determination of organic and total carbon after dry combustion (elementary analysis). International Organization for Standardization, Genebra
ISO 11260 (1994) Soil quality: determination of effective cation exchange capacity and base saturation level using barium chloride solution. International Organization for Standardization, Genebra
Karimi H, Mokhtari M, Salehi F et al (2017) Changes in microbial pathogen dynamics during vermicomposting mixture of cow manure–organic solid waste and cow manure–sewage sludge. Int J Recycl Org Waste Agric 6:57–61. https://doi.org/10.1007/s40093-016-0152-4
Kaur A, Singh J, Vig AP et al (2010) Cocomposting with and without Eisenia fetida for conversion of toxic paper mill sludge to a soil conditioner. Bioresour Technol 101:8192–8198. https://doi.org/10.1016/j.biortech.2010.05.041
Kenyangi A, Blok W (2013) Vermicompost as a component in potting mixes for growth promotion in ornamental plants. Rwanda J 28:53–63. https://doi.org/10.4314/rj.v28i0.4
Llaven MAO, Jimenez JLG, Coro BIC et al (2008) Fruit characteristics of bell pepper cultivated in sheep manure vermicompost substituted soil. J Plant Nutr 31:1585–1598. https://doi.org/10.1080/01904160802244738
Lorenzeni MZ, Rezende R, Souza AHCS et al (2015) Estimativa da área foliar em função das dimensões lineares da folha de pimentão. In: XLIV Congresso Brasileiro de Engenharia Agrícola, São Pedro
Makkar C, Singh J, Parkash C (2017) Vermicompost and vermiwash as supplement to improve seedling, plant growth and yield in Linum usitassimum L. for organic agriculture. Int J Recycl Org Waste Agric 6:203–218. https://doi.org/10.1007/s40093-017-0168-4
Malavolta E (1980) Elementos de nutrição mineral de plantas. Ceres, São Paulo
Malavolta E, Vitti GC, Oliveira SA (1997) Avaliação do estado nutricional das plantas: princípios e aplicações. POTAFOS, Piracicaba
Marti HR, Mills HA (1991) Calcium uptake and concentration in bell pepper plants as influenced by nitrogen form and stages of development. J Plant Nutr 14:1177–1185. https://doi.org/10.1080/01904169109364276
Ndegwa PM, Thompson SA, Das KC (2000) Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour Technol 71:5–12. https://doi.org/10.1016/S0960-8524(99)00055-3
NEN 5754 (1994) Determination of organic matter content in soil as loss-on-ignition. Netherlands Normalisation Institute, Delft
NHI (2012) Office of dietary supplements. Dietary supplement fact sheet: chromium. In: National Institutes of Health. http://ods.od.nih.gov. Accessed 30 Nov 2017
Nunes RR, Bontempi RM, Mendonça G et al (2016) Vermicomposting as an advanced biological treatment for industrial waste from the leather industry (tanneries). J Environ Sci Health Part B 51:271–277. https://doi.org/10.1080/03601234.2015.1128737
Oliveira JED, Marchini JS (2008) Ciências nutricionais. Sarvier, São Paulo
Pereira AL (1990) Cultura do pimentão. DNOCS, Fortaleza
Ramesh P, Singh M, Panwar NR et al (2006) Response of pigeonpea (Cajanus cajan) varieties to organic manures and their influence on fertility and enzyme activity of soil. Indian J Agric Sci 76:252–254
Ravindran B, Dinesh SL, Kennedy LJ, Sekaran G (2008) Vermicomposting of solid waste generated from leather industries using epigeic earthworm Eisenia foetida. Appl Biochem Biotechnol 151:480–488. https://doi.org/10.1007/s12010-008-8222-3
Ribeiro LG, Lopes JC, Martins Filho S, Ramalho SS (2000) Adubação orgânica na produção de pimentão. Hortic Bras 18:134–137. https://doi.org/10.1590/S0102-05362000000200012
Ricci MSF (1996) Manual de vermicompostagem. EMBRAPA Rondônia, Porto Velho
Riga P, Anza M (2003) Effect of magnesium deficiency on pepper growth parameters: implications for determination of magnesium critical value. J Plant Nutr 26:1581–1593. https://doi.org/10.1081/PLN-120022367
Scaglia B, Nunes RR, Rezende MOO et al (2016) Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci Total Environ 562:289–295. https://doi.org/10.1016/j.scitotenv.2016.03.212
Scivittaro WB, Melo AMTA, Tavares M et al (1999) Caracterização de híbridos de pimentão em cultivo protegido. Hortic Bras 17:147–150. https://doi.org/10.1590/S0102-05361999000200014
Silva MAG, Boaretto AE, Muraoka T et al (2001) Efeito do nitrogênio e potássio na nutrição do pimentão cultivado em ambiente protegido. Rev Bras Ciência do Solo 25:913–922. https://doi.org/10.1590/S0100-06832001000400014
Silva MAA, Escobedo JF, Galvani E (2002) Influência da cultura do pimentão (Capsicum annuum L.) nos elementos ambientais em ambiente protegido. Irriga 7:230–240. https://doi.org/10.15809/irriga.2002v7n3p230
Singh RP, Singh P, Araujo ASF et al (2011) Management of urban solid waste: vermicomposting a sustainable option. Resour Conserv Recycl 55:719–729. https://doi.org/10.1016/j.resconrec.2011.02.005
Swift RS (1996) Organic matter characterization. In: Sparks DL, Page AL, Helmke PA et al (eds) Methods of soil analysis: chemical methods. Soil Science Society of America, Madison, pp 1018–1020
Thor MY, Harnack L, King D et al (2011) Evaluation of the comprehensiveness and reliability of the chromium composition of foods in the literature. J Food Compost Anal 24:1147–1152. https://doi.org/10.1016/j.jfca.2011.04.006
Tobiašová E (2011) The effect of organic matter on the structure of soils of different land uses. Soil Tillage Res 114:183–192. https://doi.org/10.1016/j.still.2011.05.003
Vig AP, Singh J, Wani SH, Dhaliwal SS (2011) Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour Technol 102:7941–7945. https://doi.org/10.1016/j.biortech.2011.05.056
Vique CC, Teissedre PL, Cabanis MT, Cabanis JC (1997) Determination and levels of chromium in French wine and grapes by graphite furnace atomic absorption spectrometry. J Agric Food Chem 45:1808–1811. https://doi.org/10.1021/jf960691b
Yang J, Lv B, Zhang J, Xing M (2014) Insight into the roles of earthworm in vermicomposting of sewage sludge by determining the water-extracts through chemical and spectroscopic methods. Bioresour Technol 154:94–100. https://doi.org/10.1016/j.biortech.2013.12.023
Acknowledgements
The authors thank FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo (State of São Paulo, Brazil) for providing grants to R. R. Nunes (Process 2013/00744-0) and CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) for the support (Process 306715/2013-9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nunes, R.R., Pigatin, L.B.F., Oliveira, T.S. et al. Vermicomposted tannery wastes in the organic cultivation of sweet pepper: growth, nutritive value and production. Int J Recycl Org Waste Agricult 7, 313–324 (2018). https://doi.org/10.1007/s40093-018-0217-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-018-0217-7