Abstract
In the present study, Afzelia quanzensis bark extract was tested for the biosynthesis of silver nanoparticles (AgNPs). Based on UV–Vis spectrum analysis, the characteristic absorption band was observed at 427 nm. Furthermore, the size and shape of the nanoparticles ranged from 10 to 80 nm and were spherical in shape as observed through SEM analysis. In addition, the X-ray diffraction analysis showed that the silver nanoparticles are crystalline in nature and have a face-centred cubic structure. Based on FTIR analysis, the presence of phytochemical functional groups such as carboxyl (–C=O) and amine (N–H) in Afzelia quanzensis bark extract further confirmed the responsible reducing agents for nanoparticles formation. Interestingly, the synthesized silver nanoparticles at 50 mg/L concentration showed significant antibacterial activity against Escherichia coli and Staphylococcus aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, nanotechnology is a rapidly developing field of importance since it deals with the synthesis and stabilization of different metal nanoparticles. Among the particles, silver nanoparticles (AgNPs) have become the focus of intensive research due to several important applications such as their use in bio-labelling, sensors, drug delivery system, antimicrobial agents and filters [1, 2]. The synthesized silver nanoparticles exhibit new or improved properties depending upon their size, morphology and distribution [3]. The production of pure and well-defined metal-based nanoparticles by chemical reduction [4], thermal treatment, irradiation [5] and laser ablation [6] have been reported. On one hand, organic solvents, toxic reducing agents, high-pressure and high-temperature conversion which are potentially dangerous to the environment are used [7]. Hence, a pressing need to shift from physical and chemical synthesis to ‘green’ chemistry and bioprocesses is of major interest.
In the last decade, the biosynthesis of nanoparticles has received increasing attention due to the growing need to develop environmentally benign technologies in material synthesis [8, 9]. Biological routes for the synthesis of metal nanoparticles by exploiting bacteria [10, 11], marine fungus Penicillium fellutanum [1], fungus Aspergillus foetidus [12], yeast [13, 14], enzymes [15] have been reported. However, one of the major drawbacks in using microbes for nanoparticle synthesis is the elaborate process of maintaining microbial cultures. The use of plant extracts for the synthesis of nanoparticles have gained momentum in recent years and could be advantageous over other environmentally benign biological processes by eliminating the elaborate process of maintaining cell cultures, being simply, eco-friendly and this could be an exciting possibility that is relatively unexplored and under exploited [16]. Green silver nanoparticles synthesis using various natural products like Magnolia kobus [17], Acacia leucophloea extract [18], Zizyphus xylopyrus bark extract [19], aloevera plant extract [20, 21], Cinnamon zeylanicum bark extract [22], Curcuma longa tuber powder [23] and Jatropha curcas [9] have been reported. However, the potential of other plants as sources of biomaterial for the synthesis of new nanoparticles are yet to be entirely explored.
It has been reported that plants contain different phytochemical products [24] which are able to breakdown the silver nitrate, a complex hazardous chemical into Ag+ and NO3 − ions. In the process, the toxic Ag+ ions are further reduced to the nontoxic (Ag0) metallic nanoparticles through the use of different functional groups on the surface of the extract [25]. In the present study, we selected Afzelia quanzensis, Pod mahogany (English), Mujarakamba (Shona name in Zimbabwe) bark as a biomaterial for supplying the different phytochemicals required for the synthesis of silver nanoparticles and the mechanism involved in the synthesis is illustrated in Fig. 1. The plant is economic and abundantly available in Zimbabwe. It is a medium-sized to large deciduous tree and has a bark which is greyish-brown, flaking and leaving pale patches [26]. It produces fruits which consist of a large flattened pod, thickly woody, 10–17 cm, and splitting to reveal large, shiny black seeds with a bright red aril. In medicine, roots are used to treat gonorrhoea, chest pains, kidney problems, bilharzia, eye problems and snakebites, and a small piece of bark is applied to an aching tooth. The plant can be categorized as:
Taxonomy
Kingdom: Plantae
Division: Tracheophyta
Subdivision: Spermatophytina
Class: Magnoliopsida
Order: Fabales
Family: Fabaceae
Genus: Afzelia Sm.—mahogany
Species: Afzelia quanzensis Welw.—pod mahogany
In the present study, we used plant bark extracts for synthesis of silver nanoparticles by monitoring their conversion using UV–visible spectroscopy. We also investigated the effects of reaction conditions such as temperature, pH, quantities of bark extract and AgNO3 concentration on the synthesis rate. The silver nanoparticles were further characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectrometer, and Fourier transform infrared spectroscopy (FTIR). Furthermore, the antibacterial activity of the silver nanoparticles on a strain of Escherichia coli and Staphylococcus aureus was qualitatively evaluated by the zone inhibition method.
Materials and methods
Preparation of Afzelia quanzensis bark powder and extract
The bark was obtained from Afzelia quanzensis tree in Chivi rural district area, Zimbabwe. The bark was washed to remove any impurities and dried under sunlight for a week to completely remove the moisture. The bark was cut into small pieces, powdered in a mixer and then sieved using a 20-mesh sieve to get uniform size range. The sieved powder was used for all further studies. For the production of an extract, 50 g of powdered bark was added to a 500-mL Erlenmeyer flask containing 200 mL deionized water and then boiled for 15 min. After cooling, the mixture was filtered through Whatman filter paper no. 1 and the extract was kept at 4 °C prior to silver nanoparticles synthesis.
Synthesis of silver nanoparticles
Silver nitrate (AgNO3) analytical grade used as a precursor was purchased from Sigma-Aldrich (Pretoria, South Africa). For the AgNPs synthesis, 5 mL bark extract were added to 50 mL of 1 mM aqueous AgNO3 solution in a 250-mL Erlenmeyer flask. The flask was then incubated in a rotary shaker at 160 rpm in the dark. The reduction of silver ions was routinely monitored visually for colour change at regular intervals. Thereafter, the silver nanoparticle solution thus obtained was purified by repeated centrifugation at 500 rpm for 20 min followed by re-dispersion of the pellet of silver nanoparticles into a 10 mL of deionized water. After freeze drying, lyophilization process was performed on the purified silver nanoparticles to obtain the powdered form which was stored in brown bottles prior to physical characterization and antibacterial activity.
Characterization
UV–Vis spectrophotometer analysis
The preliminary reduction of synthesized silver nanoparticles was monitored using UV–visible spectrophotometer (Shimadzu UV-1601, Japan) by scanning the absorbance spectra in the range of 300–600 nm. All sample solutions were diluted using a ratio of 1:10.
Electron microscopic study
SEM analysis of the synthesized silver nanoparticles was done using a Hitachi S-4500 SEM machine (Japan). A thin layer of gold was used to coat the samples through vacuum evaporation so that the nanoparticles conducts evenly and provides a homogeneous surface for analysis and imaging on an aluminium slab. The elemental analysis was performed using EDX (JEOL-JSM-5800LV, Tokyo, Japan) which is an attachment to the SEM. The sample powder of AgNPs was compressed to form tablets before analysis with EDX spectrum.
XRD analysis
The dried synthesized silver nanoparticles were analysed using an X-ray diffractometer (D8 Bruker, Germany) with Cu K α radiation in the range of 30o ≤ 2θ ≤ 90° at 40 kV and 40 mA.
FTIR analysis
FTIR (PerkinElmer, US) spectra were obtained at room temperature in the spectral range between 480 and 4000 cm−1. FTIR measurements were made to identify the possible biomolecules responsible for capping and efficient stabilization of the synthesized silver nanoparticles.
Study for the influence of different parameters
Influence of different temperatures (30, 40, 50, 60, 70, 80, 90 °C), pH values (3, 5, 7, 8, 11), bark extract amounts (2, 4, 6, 8, 10, 12, 14 mL), substrate concentrations (1, 3, 5, 7, 9, 11, 13 mM AgNO3) and incubation periods (20, 40, 60, 80, 100, 120, 140 min) were investigated by varying the parameters one at a time. A sample of 1 mL was withdrawn at different time intervals and the absorbance was measured at 427 nm.
Measuring concentration of AgNPs using inductively coupled plasma optical emission spectrophotometer (ICP-OES)
The original concentration (80 mg/L) of the Afzelia quanzensis bark extract mediated AgNPs was measured using ICP-OES. Then, by diluting this solution, samples of different concentrations (10, 25, 50 mg/L) were used to investigate the concentration dependence of the antibacterial effect of Ag nanoparticles.
Evaluation of antibacterial activity of synthesized silver nanoparticles
The synthesized silver nanoparticles were tested for their antibacterial activity by using the disk diffusion method. The cultures of Staphylococcus aureus (ATCC-25923) and Escherichia coli (ATCC-39403) were obtained from American Type Culture Collection Center, USA. S. aureus and E. coli were grown on Mueller–Hinton agar medium. The disk diffusion was performed by placing different types of disks including bark extract (50 μL), synthesized silver nanoparticles (50 μL/10, 25, 50 mg/L), standard antibiotic (erythromycin 50 μL) and synthesized silver nanoparticles with standard antibiotic on the surface of the agar in plates and incubated for 24 h at 37 °C. The zones of inhibition were measured by the Hi-Media scale.
Results and discussion
Visual analysis
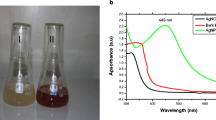
Afzelia quanzensis bark extract when incubated with AgNO3 solution under dark conditions changed its colour from colourless to light reddish and finally to reddish brown. The colour of the filtrate changed to intense brown after 120 min of incubation (Fig. 2b, c). The control AgNO3 solution (without Afzelia quanzensis bark extract) showed no change in colour (Fig. 2a). The possible chemical reactions involved in the preparation of the AgNPs can be represented as:
UV–Vis spectra analysis
The development of metal silver nanoparticles in aqueous solution was described by UV–Vis spectroscopy. The absorption spectra of AgNPs formed at different durations are shown in (Fig. 3).
From Fig. 3, it can be observed that the plasmon band was symmetric, indicating that the solution does not have much aggregated particles [27]. The evolution of an absorption spectra for the AgNPs shows an increasingly sharp absorbance at 427 nm with increase in time, which steadily increased in intensity as a function of reaction time without showing any shift of the maximum wavelength [2]. This phenomenon may be linked to polarization of the free conduction electrons with respect to the much heavier ionic core of AgNPs, resulting in electron dipolar oscillation after exposure of AgNPs to light [12]. For quality assurance and comparison, AgNO3 solution in deionized water showed no absorption peak at the same wavelength range (data not shown). In the present bark extract/Ag investigation, the reaction mixtures showed a single SPR band revealing the spherical shape of AgNPs which is in good agreement to the Mie’s theory [28]. Consequently, this validates the application of Afzelia quanzensis bark extract as a precursor for AgNPs synthesis.
Study for the influence of different parameters
The biosynthesis of AgNPs is affected by a variety of factors (substrate concentration, electron donor, incubation time, pH, temperature, buffer strength, etc.) which control the shape and size as well as achieving the monodispersity in solution phase. In this study, the effects of pH, AgNO3 concentrations, different quantities of bark extract, and different temperatures were investigated.
Effect of pH
The size and morphology of nanoparticles are mainly affected by the pH of solution [29–31]. As shown by UV–Vis spectroscopy (Fig. 4), when the pH of the reaction mixture was increased, an increase in absorbance was observed. This might be due to the increase in production of colloidal silver nanoparticles and reduction rate. Visual observation showed the amount of nanoparticles to be pH value dependent. The reaction mixture colouring accelerated when the pH was increased. At acidic pH, large-sized silver nanoparticles were observed, whilst at higher pH highly dispersed, small-sized nanoparticles were formed. The results are in agreement with those reported in literature [32–34]. Control experiments (AgNO3 solution incubated at different alkaline pH 8, 9, 10) showed no synthesis of nanoparticles.
Effect of AgNO3 concentration
The concentration of AgNO3 which might be converted to a final product is one of the important measures required to make the reaction more economical and efficient [31]. The effect of AgNO3 concentration on synthesis of AgNPs is shown in Fig. 5.
There was an increase in synthesis of AgNPs with respect to Ag+ ion concentration in the range 1–9 mM. However, the absorbance was found to decrease at concentrations greater than 9 mM. Comparable results were obtained for the synthesis AgNPs using Pinus eldarica bark extract [31]. Consequently, 9 mM was used for further studies.
Effect of different quantities of Afzelia quanzensis bark extract
The production of AgNPs was monitored as a function of different amounts of Afzelia quanzensis bark extract amount. Figure 6 shows the effect of extract amount on AgNPs production. The increase in the extract amount from 2 to 10 mL caused a considerable increase in peak absorbance in UV–Vis spectrum. The increase in the amount of soluble phytochemical reducing agents in the extract would mean more Ag+ ion reduction, and subsequently more nanoparticle production. Furthermore, a decrease in amount of Ag nanoparticles was observed due to an increase in extract amount above 10 mL.
Effect of temperature
The role of temperature on the reaction rate from 30 to 70 °C was investigated. An increase in absorbance was noted as the temperature increased (Fig. 7). The enhanced rate of synthesis of AgNPs might be due to the reaction temperature increasing the kinetic energy of the reacting molecules thus more Ag+ ions were in collision with the reducing molecules of the extract. The maximal synthesis of AgNPs was achieved at 70 °C.
Characterization
Electron microscopic study
The SEM images of the AgNO3 (Fig. 8a) and synthesized silver nanoparticles (Fig. 8b) were clearly noticeable. The size of the silver nitrate particles used as control in this study was greater than 1 000 nm size (Fig. 8a); whereas, the synthesized silver nanoparticles measured 10–80 nm in size (Fig. 8b). Similar to our study, the same pattern of silver nanoparticles were also reported [24]. The EDAX spectroscopy results confirmed the significant presence of 61.66 % silver, 30.47 % carbon and oxygen 7.87 % (Fig. 9). Metallic silver nanocrystals generally show a typical optical absorption peak approximately at 3 keV due to surface plasmon resonance [35]. The weak signals at 0.25 and 0.50 keV were are for carbon and oxygen, respectively, which might arise from the functional compounds present in the aqueous extract.
XRD: purity and crystalline nature of AgNPs
The phase of the vacuum dried nanoparticles was investigated by XRD and corresponding patterns are shown in Fig. 10. However, silver nanoparticles have shown clear peaks of cubic phases (JCPDS No. 03-0921) at 38.1 (111), 42.9 (200), 65.5 (220) and 77.9 (311). XRD pattern thus clearly illustrates that the silver nanoparticles formed in this present synthesis are crystalline in nature. There were no other corresponding peaks observed from the XRD pattern which showed that the formed AgNPs have a high purity. Previous studies have also reported the crystalline nature of biosynthesized AgNPs using different plant extracts [3, 9, 22, 23, 36]. The average crystalline size of silver nanoparticles synthesized using Afzelia quanzensis bark extract can be calculated using the Scherrer equation [25]:
where D is the crystallite size of AgNPs, λ is the wavelength of the X-ray source (0.1541 nm), β is the full width at half maximum of the diffraction peak, K is the Scherrer constant with a value from 0.9 to 1, and θ is the Bragg angle. The average crystalline size was 19.8 nm. The obtained average crystalline size coupled with the presence of structural peaks in XRD patterns clearly illustrated that the AgNPs synthesized were nanocrystalline in nature.
FTIR analysis
The FTIR spectra of Afzelia quanzensis extract and synthesized AgNPs were examined (Fig. 11). The strong band at 3455 cm−1 on the Afzelia quanzensis extract is characteristic of N–H and O–H stretching vibrations [35]. The characteristic absorption band at 2933 cm−1 is due to alkyl chains. The FTIR spectra also show bands at 1637 and 1445 cm−1 identified as amide I and amide II which arise due to carbonyl (C=O) and amine (–NH) stretching vibrations in the amide linkages of the proteins, respectively. The peaks at 1264 and 1060 cm−1 may be due to a carboxylate group (COO−) and phosphate group, respectively. After reduction of AgNO3, the band characteristic of N–H and O–H stretching vibrations shifted to 3424 cm−1 and alkyl group decreased in intensity, and shifted to 2937 cm−1. The disappearance of the peaks at 1445 and 1264 cm−1 and formation of new intense peak at 1384 cm−1, signify the involvement of the secondary amines in the reduction process. The shift of the band from 1635 to 1637 cm−1 is attributed to the binding of (NH) CO group with nanoparticles. It can be concluded from the FTIR study that the carboxyl (–C=O) and amine (N–H) groups in Afzelia quanzensis bark extract were mainly involved in reduction of Ag+ ions to Ag0 nanoparticles.
Stability studies
A vital aspect in colloid chemistry is how nano-scale particles are stabilized in their reaction media as smaller particles are prone to agglomeration. The stability of the AgNPs in solution was checked using UV–Vis spectroscopy by observing changes in the SPR band peak [37]. Figure 12 shows UV–Vis spectra periodically obtained over 60 days. The AgNPs produced from bark extract were observed to be very stable in solution when monitored periodically over a period of 40 days; with no evidence of flocculation or change in SPR, measured at 427 nm. However, after 50 days the surface plasmon resonance absorption band decreased and can be attributed to destabilization of AgNPs in solution due to aggregation. Between 50 and 60 days secondary peaks appear at longer wavelength which is a sign of destabilization.
Evaluation of antibacterial activity of synthesized silver nanoparticles
The antimicrobial activity of bark extract (a), synthesized AgNPs of different concentrations (b–d), the antibiotic (erythromycin) (e) and antibiotic plus different concentrations of synthesized AgNPs (f–h) were evaluated by diffusion method. The bacterial cultures used were Gram-positive bacteria, i.e. Staphylococcus aureus and Gram-negative bacteria, i.e. Escherichia coli. The increase in zone of inhibition of silver nanoparticles at different concentrations (10, 25, 50 mg/L) compared with antibiotic for both bacterial cultures (Fig. 13a, b) demonstrated the lesser antibacterial potential of silver nanoparticles with that of antibiotics. The zone of inhibition also increased with AgNPs concentration in both cultures. The bark extract exhibits the highest activity against E. coli than S. aureus. The obtained results support, at least in part, the use of this plant as traditional medicine against Gram-negative bacteria due to the presence of phytochemicals. Furthermore, the phytofabricated silver nanoparticles were found to be more effective against Escherichia coli as compared to that of Staphylococcus aureus. From Fig. 13a, b, it can be confirmed that combining the antibiotics with AgNPs resulted in a greater bactericidal effect on test pathogens than either of the antibacterial agents used alone. Inhibition of Escherichia coli might have been facilitated by silver’s high affinity for phosphorus and sulphur which are part of the cell membranes of Gram-negative bacteria [38]. The sulphur and phosphorus reacts with AgNPs causing dysfunction of enzymes on bacteria cell wall and also it disturbs DNA’s moieties process thereby denying replication [39]. On the other hand, the cell wall of Gram-positive bacteria is much more rigid due to the presence of a thick peptidoglycan layer, which is superficial to the cell membrane hence a difference in activity observed.
Graphical representation of inhibition zones by plant extract (a), 10 mg/L synthesized AgNPs (b), 25 mg/L synthesized AgNPs (c), 50 mg/L synthesized AgNPs (d), erythromycin (e), erythromycin plus 10 mg/L synthesized AgNPs (f), erythromycin plus 25 mg/L synthesized AgNPs (g), erythromycin plus 50 mg/L synthesized AgNPs (h), for a Escherichia coli, b Staphylococcus aureus (error bar ±SD and n = 3)
Conclusion
The silver nanoparticles were green synthesized using bark extract of Afzelia quanzensis. The method represents an example of clean, nontoxic and eco-friendly method for obtaining silver nanoparticles. Colour changes occur due to surface plasmon resonance during the reaction with Afzelia quanzensis bark extract resulting in the formation of silver nanoparticles, which was confirmed by XRD, FTIR, UV–Vis spectroscopy, and EDAX. The silver nanoparticles were found to be stable in water for 40 days. The zone of inhibition test showed that the synthesized nanoparticles have some antibacterial activity. Further studies will be conducted to isolate and quantify the different phytochemical components and to study their pharmacological properties after synthesizing the silver nanoparticles from the specific phytochemicals.
References
Kathiresan K, Manivannan S, Nabeel MA, Dhivya B (2009) Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf 71:133–137
Sridhara V, Pratima K, Krishnamurthy G, Sreekanth B (2013) Vegetable assisted synthesis of silver nanoparticles and its antibacterial activity against two human pathogens. Asian J Pharm Clin Res 6:53–57
Awwad AM, Salem NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Intern J Indust Chem 4:29–35
Peterson MSM, Bouwman J, Chen Deutsch AM (2007) Inorganic metallodielectric materials fabricated using two single-step methods based on the Tollen’s process. J Colloid Interface Sci 306:41–49
Shao K, Yao J (2006) Preparation of silver nanoparticles via a non-template method. Mater Lett 60:3826–3829
Tsuji T, Iryo K, Watanabe N, Tsuji M (2002) Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl Surf Sci 202:80–85
Nagaraz B, Krishnamurthy NB, Liny P, Divya TK, Dinesh R (2011) Biosynthesis of gold nanoparticles of Ixora coccinea flower extract and their antimicrobial activities. Int J Pharma Bio Sci 2:557–565
Bar H, Bhui DK, Sahoo GP Sarkar P, De SP, Misra A (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf 339:134–139
Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A 348:212–216
Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area3. Biotech 4(2):121–126
Ghorbani HR (2013) Biosynthesis of silver nanoparticles using Salmonella typhirium. J Nanostruct Chem 3(29):1–4
Swarup R, Kumar DT (2014) Biosynthesis of silver nanoparticles by Aspergillus foetidus: optimization of physicochemical parameters. Nanosci Nanotechnol Lett 6(3):181
Prasad K, Anal KJ, Kulkarni AR (2007) Lactobacillus assisted synthesis of titanium nanoparticles. Nanoscale Res Lett 2:248–250
Jha AK, Kamalesh P, Prasad K (2009) A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem Eng J 43:303–306
Hebbalalu D, Lalley J, Nadagouda MN, Varma RS (2013) Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain Chem Eng 1(7):703–712
Prathap M, Alagesan A, Ranjitha Kumari BD (2014) Anti-bacterial activities of silver nanoparticles synthesized from plant leaf extract of Abutilon indicum (L.) Sweet. J Nanostruct Chem 4:106
Lee SH, Salunke BK, Kim BS (2014) Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts. Biotechnol Bioprocess Eng 19:169–174
Murugan K, Senthilkumar B, Senbagam D, Al-Sohaibani S (2014) Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int J Nanomed 9:2431–2438
Maria BS, Devadiga A, Kodialbail VS, Saidutta MB (2015) Synthesis of silver nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl Nanosci 5:755–762
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using aloevera plant extract. Biotechnol Progress 22:577–583
Sharma NC, Sahi SV, Nath S, Parsons JG, Gardea-Torresdey JL, Pal T (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix embedded nanomaterials. Environ Sci Technol 41:5137–5142
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B 73:332–338
Sathishkumar M, Sneha K, Yun YS (2010) Immobilization of silver nanoparticles synthesized using curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour Technol 101:7958–7965
Bhati-Kushwaha H, Malik CP (2014) Biosynthesis of silver nanoparticles using fresh extracts of Tridax procumbens Linn. Indian J Exp Biol 52:359–368
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B 81:81–86
Biegel HM (1977) Check-list of ornamental plants used in Rhodesian parks and gardens. Rhod Agric J Res Rep 3:19–20
Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103:8410–8426
Ghadimi A, Saidur R, Metselaar HSC (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54:4051–4068
Konishi Y, Tsukiyama TTT, Saitoh N, Nomura T, Nagamine S (2007) Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta 53:186–192
Sanghi R, Verma P (2009) Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol 100:501–504
Iravani S, Zolfaghari B (2013) Green synthesis of silver nanoparticles using pinus bark extract. Biomed Res Int 2013:1–5
Gardea-Torresdey JL, Tiemann KJ, Gamez G, Dokken K, Tehuacanero S, Jos´e-Yacam´an M (1999) Gold nanoparticles obtained by bio-precipitation from gold (III) solutions. J Nanopart Res 1:397–404
Birla SS, Gaikwad SC, Gade AK, Rai MK (2013) Rapid synthesis of silver nanoparticles from fusarium oxysporum by optimizing physicocultural conditions. Sci World J 2013:1–12. doi:10.1155/2013/796018
Armendariz V, Herrera I, Peralta-Videa JR (2004) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6:377–382
Das SK, Khan MMR, Guha AK, Das AR, Mandal AB (2012) Silver-nano biohybride material: synthesis, characterization and application in water purification. Bioresour Technol 124:495–499
Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B 76:50–56
Lee DK, Tho NTM, Minhan TN, Tri MD, Sreekanth TVM, Lee JS, Nagajyothi PC (2013) Green synthesis of silver nanoparticles using Nelumbo nucifera seed extract and its antimicrobial activity. Acta Chim Slov 60:673–678
Hari N, Thomas TK, Nair AJ (2014) Comparative study on the synergistic action of differentially synthesized silver nanoparticles with β-Cephem antibiotics and chloramphenicol. J Nanosci 2014:1–8
Shetty P, Supraja N, Garud M, Prasad TNVKV (2014) Synthesis, characterization and antimicrobial activity of Alstonia scholaris bark-extract-mediated silver nanoparticles. J Nanostruct Chem 4(4):161–170
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moyo, M., Gomba, M. & Nharingo, T. Afzelia quanzensis bark extract for green synthesis of silver nanoparticles and study of their antibacterial activity. Int J Ind Chem 6, 329–338 (2015). https://doi.org/10.1007/s40090-015-0055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0055-7