Abstract
In the present paper, biosynthesis of silver nanoparticles using Zizyphus xylopyrus bark extract is reported. Z. xylopyrus bark extract is efficiently used for the biosynthesis of silver nanoparticles. UV–Visible spectroscopy showed surface plasmon resonance peaks in the range 413–420 nm confirming the formation of silver nanoparticles. Different factors affecting the synthesis of silver nanoparticles like methodology for the preparation of extract, concentration of silver nitrate solution used for biosynthesis and initial pH of the reaction mixture were studied. The extract prepared with 10 mM AgNO3 solution by reflux extraction method at optimum initial pH of 11, resulted in higher conversion of silver ions to silver nanoparticles as compared with those prepared by open heating or ultrasonication. SEM analysis showed that the biosynthesized nanoparticles are spherical in nature and ranged from 60 to 70 nm in size. EDX suggested that the silver nanoparticles must be capped by the organic components present in the plant extract. This simple process for the biosynthesis of silver nanoparticles using aqueous extract of Z. xylopyrus is a green technology without the usage of hazardous and toxic solvents and chemicals and hence is environment friendly. The process has several advantages with reference to cost, compatibility for its application in medical and drug delivery, as well as for large-scale commercial production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
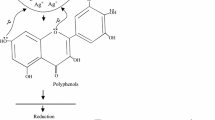
Nanoparticles are extremely small structures, the synthesis of which is much more complicated than that of their macroscopic counterparts. Research in the field of nanotechnology has gained significant momentum as the properties demonstrated by these structures at the nanoscale level are remarkable in terms of their applicability (Duncan 2011). Silver nanoparticles have been the prime focus of the nanoparticles’ research industry due to their unique thermal (Moon et al. 2005), electrical (Chen et al. 2009) and optical (Kelly et al. 2003) properties and also because of the use of these structures in products that range from photovoltaics (Yoon et al. 2010) to biological and chemical sensors (McFarland and Van Duyne 2003). Also nanosilver has been used extensively as an anti-bacterial agent in the health industry (Jain and Pradeep 2005), food storage (Costa et al. 2011), textile coatings (Perelshtein et al. 2008) and also for a number of environmental applications (Li et al. 2008). Currently nanosilver is prepared by different methods including electrolysis, physical, chemical, and biological methods (Dubey et al. 2010; Usha Rani and Reddy 2011). Biological methods are the biomimetic methods which refer to the application of biological principles in material formation. Bioreduction of a precursor salt of silver by the bioactive compounds present in plants is one such biomimetic method in which, natural products are used as reducing agents (Bhainsa and D’Souza 2006). Biosynthesis of silver nanoparticles using plant extracts may be influenced directly or indirectly by phytochemicals in extracts such as phenols, flavonoids and antioxidants as well as the physicochemical factors governing the kinetics of the reaction. This route is preferably docile as it is ecofriendly, involves less energy intensive processes and is cost effective. Moreover, it is an efficient way of waste biomass utilization for the biosynthesis of silver nanoparticles.
Biosynthesis of silver nanoparticles has been carried out successfully using the extracts of plants such as, Lippia citriodora (Cruz et al. 2010), Mollugo nudicaulis (Anarkali et al. 2010), Prunus armeniaca (Dauthal and Mukhopadhyay 2013), Azadirachta indica (Renugadevi and Venus 2012), Desmodium gangeticum (L.) DC. (Thirunavoukkarasu et al. 2013) Citrus sinensis (Kaviya et al. 2011), Mangifera indica (Philip 2011), Alternanthera dentata (Kumar et al. 2012) Murrayya koenigii (Philip et al. 2011), Magnolia kobus (Kim et al. 2014), Malva parviflora (Mervat et al. 2012) and Ocimum Tenuiflorum (Patil et al. 2012a). Zizyphus xylopyrus (Family: Rhamnaceae) is found throughout northwestern India, Pakistan and China, a large, straggling shrub or a small tree, armed with spines. The major chemical compositions of Z. xylopyrus are Quercetin, Kempferol-4’-methylether and Kempferol, Cyclo peptide alkaloids Amphibine-H and Nummularine-K (Vimal et al. 2009). The root bark of this plant is reported to have an anticonvulsant and anti-inflammatory activity (Kumar et al. 2011). It also possess anxiolytic, anticancer, antifungal, antibacterial, antiulcer, anti-inflammatory, antispastic, and wound healing properties (Saima et al. 2013). As it is rich in flavonoids, it can serve as an ideal candidate for reduction of silver salt to nanosilver.
The current paper presents the biosynthesis of silver nanoparticles by bioreduction of the precursor salt using extracts of the stem bark of Z. xylopyrus. The conditions under which the biosynthesis occurs have also been studied. To the best of our knowledge, this is the first study that uses Z. xylopyrus bark extract for the synthesis of silver nanoparticles.
Materials
Silver nitrate was purchased from Merck Specialities Private Ltd., India and used as received. Z. xylopyrus stem barks were purchased from a local market in Surathkal, Karnataka, India.
Methods
Preparation of plant extract
Zizyphus xylopyrus bark was thoroughly washed with tap water followed by a series of rinses with distilled water to remove any impurities and dried in shade for a week to completely remove the moisture. The bark was cut into small pieces, powdered in a motor operated domestic mixer and stored in airtight dark bottles for further use. For the preparation of extract three different methods of (1) open heating, (2) reflux extraction and (3) ultrasonication extraction were used. (a) For extraction by open heating, 5 g of the stored powder was mixed with 100 ml of distilled water and heated until boiling and then boiled for a duration of 5 min. (b) For reflux extraction, 5 g of the plant bark powder was mixed with 100 ml of distilled water and extraction with water was carried out at a temperature of 100 °C under reflux condition for a period of 1 h. (c) For extraction by ultrasonication, the slurry of 5 g of the plant bark powder mixed with 100 ml of distilled water was subjected to sonication for a time period of 10 min under conditions of 90 % amplitude and at the rate of 20 pulses per second using Vibra Cell (Sonics, USA) probe sonicator. The bark decoction hence obtained after the extraction was allowed to cool till 28 ± 2 °C filtered using Whatman No. 1 filter paper and stored at 4 °C for future use.
Preparation of silver nitrate solution
Different concentrations of silver nitrate solutions (1–100 mM) were prepared and stored in amber-colored bottles.
Biosynthesis of silver nanoparticles
For biosynthesis of silver nanoparticles, 1 ml of the prepared extract was added to 4 ml of 1 mM aqueous silver nitrate solution. The reaction was carried at 28 ± 2 °C for a period of 24 h. The visual colour change in the reaction mixture from light yellow to dark brown was observed at regular intervals with reference to control. The formation of silver nanoparticles was confirmed by spectrophotometric determination.
UV–Visible spectrophotometric studies on biosynthesized silver
The biosynthesis of silver nanoparticles was confirmed by carrying out spectral studies on the reaction mixture after 24 h of reaction. Spectrum studies were carried out in the range of 350–700 nm at a resolution of 1 nm using dual wavelength UV–Visible spectrophotometer (Labomed, USA).
Studies on the factors affecting the biosynthesis of silver nanoparticles
Effect of concentration of silver nitrate
To study the effect of concentration of silver nitrate solution on biosynthesis of Ag nanoparticles, during the synthesis, 4 ml of different concentrations of silver nitrate (1, 5, 10, 20, 50, 70 and 100 mM) was taken in different test tubes. 1 ml of extract was added to each of the test tubes and the reaction was allowed to occur at 28 ± 2 °C over duration of 24 h. Formation of silver nanoparticles was confirmed through observation of surface plasmon resonance (SPR) by UV–Visible spectrophotometric studies.
Effect of pH
In order to study the effect of pH, biosynthesis of nanoparticles was carried out at three different initial pH’s of 3, 7 and 11. The biosynthesis was carried out according to the procedure explained in “Biosynthesis of silver nanoparticles” but with silver nitrate at optimum concentration obtained using the results of experiments performed as per “Effect of concentration of silver nitrate” section. The pH was adjusted by using 0.1 N HNO3 and 0.1 N NaOH.
Recovery of silver nanoparticles
The colloidal reaction mixtures containing the nanoparticles were centrifuged at 15,000 rpm for 15 min. The pellets thus obtained after centrifugation were washed with 70 % ethanol and dried in hot air oven at a temperature of 120 °C for 24 h. The dried powder was separated carefully from centrifuge tubes and stored in sample vials for further analysis. The supernatant after centrifugation was analyzed for residual silver content using atomic absorption spectroscopy (AAS) for determination of conversion of silver ions to silver nanoparticles.
Conversion of silver ions to silver nanoparticles
After recovery of silver nanoparticles, concentration of residual silver ions in the supernatant was analyzed using atomic AAS (GBC 932 plus) and there by conversion of silver nitrate to silver was calculated. AAS was operated with fuel flow rate of 2 (l/min) and air flow rate of 10 (l/min) running at 338.3 nm. The conversion of silver ions to silver was calculated using Eq. (1)
where X denotes the conversion of silver ions to silver nanoparticles; C0 is the initial concentration of silver ions in the solution; and Cf is the final concentration of silver ions in the solution (Jiale et al. 2011).
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis of the biosynthesized silver nanoparticles
Silver nanoparticles biosynthesized under optimum conditions (concentration of 10 mM silver nitrate and at alkaline initial pH) using Z. xylopyrus bark extract, were recovered after centrifugation at 15,000 rpm for 15 min and the solid portion after removal of supernatant, was washed with 70 % ethanol and then dried in hot air oven at a temperature of 120 °C for 24 h. The silver nanoparticles hence obtained, were drop coated onto carbon tapes and gold sputtering (JFC-1600 Auto fine coater, JEOL, Japan) was performed before carrying out the SEM analysis. EDX analysis was performed to confirm the elemental composition of the nanoparticles.
Results and discussions
The efficacy of the extract of Z. xylopyrus to reduce silver nitrate to form silver nanoparticles was tested by using the extract prepared by open heating method. UV–Vis absorption spectroscopy is one of the most widely used simple and sensitive techniques for the observation of metal nanoparticle synthesis. Synthesis of silver nanoparticles was carried out by the methodology described in “Biosynthesis of silver nanoparticles”, using the extract prepared by open heating method and aliquots were taken from the reaction mixture and analyzed for surface plasmon peak by spectrophotometric analysis. In order to monitor the formation of silver nanoparticles, the absorption spectra of the biosynthesized silver nanoparticles were recorded against distilled water as the blank. UV absorption spectra of biosynthesized silver nanoparticles demonstrated SPR peaks characteristic of silver nanoparticles. The SPR spectra of biosynthesized silver nanoparticles obtained from the reaction medium containing Z. xylopyrus extract prepared with open heating and silver nitrate solution of 1 mM concentration is shown in Fig. 1, which has its λmax at 414 nm and broadening of the peak indicates that the nanoparticles are polydispersed. Shifting of peak from the standard 420 nm corresponding to silver nanoparticles may be attributed to the presence of bioactive components as capping agents on the surface of nanoparticles. The surface plasmon peak confirms the formation of silver nanoparticles and hence the bark extract of Z. xylopyrus is shown to have the ability to reduce silver ion to nano Ag in zerovalent state. This simple process for the biosynthesis of silver nanoparticles using aqueous extract of Z. xylopyrus is a green technology with no use of hazardous and toxic solvents and chemicals and hence is environment friendly and economical.
Factors affecting the biosynthesis of silver nanoparticles
Different factors affecting the conversion of silver ions to silver nanoparticles by biosynthesis using the extracts prepared by each of the different extraction methods (open heating, reflux extraction and ultrasonication extraction) were studied. The effect of parameters such as precursor salt concentration and initial pH upon the biosynthesis was also studied.
Effect of silver nitrate concentration
Synthesis of silver nanoparticles was carried out as described in “Biosynthesis of silver nanoparticles” with different concentrations of AgNO3 solution added to the extract. Visual confirmation of the synthesis of silver nanoparticles was obtained by the development of the characteristic brown colour of the reaction mixtures in each case. The extracts obtained by different methods were added to increasing concentration of AgNO3 solution from 1 to 100 mM and the nanoparticles were synthesized. The synthesized nanoparticles demonstrated SPR peaks characteristic of silver nanoparticles as shown in the Fig. 2a–c. Figure 2a and b show the UV–Vis spectra of nanoparticles synthesized (Sastry et al. 1998) with extracts prepared by open heating and ultrasonication. In both the cases, the synthesis mixture prepared with 10 mM silver nitrate solution showed SPR peak at 418 nm and the peak intensity increased with the increase in silver nitrate concentration from 1 to 10 mM indicating faster rate of bioreduction with increased concentration of precursor salt (Zaki et al. 2011). The rise in peak intensities with increasing salt concentration was also observed by Dubey et al. (2010). The rise in SPR peak intensities is due to the longitudinal vibrations (Prathna et al. 2011). But further increase in silver nitrate concentration to 20 mM and above lead to peaks with lower intensity which can be accounted by the formation of agglomerated nanoparticles and their settling. The settling of particles at higher concentrations may be due to the presence of large number of silver particles in small volume of solution which creates higher coalescence between silver nanoparticles and hence results in agglomeration and formation of particles with large size at micro scale. The presence of very large particles was the reason of faster settling of particles (Safekordi et al. 2011). Reaction mixtures prepared with 50 and 100 mM silver nitrate solutions demonstrated broadened peaks with less intensity because of the settling of particles.
In case of extract prepared by reflux method, synthesis increased with the increase in concentration of silver nitrate from 5 to 10 mM, indicated by the rise in SPR peak intensity as shown in Fig. 2c. Lower precursor salt concentration of 1 mM, did not display an SPR peak; this may be due to inadequate number of silver ions for bioreduction in the sample. From Fig. 2a–c it can be deduced that owing to highest intensity obtained with the synthesis mixture prepared with 10 mM of silver nitrate solution, the optimum concentration of the salt required for formation of stable colloidal nanoparticles is 10 mM for biosynthesis using extracts prepared by all the three different methods.
Effect of pH
Effect of initial pH on the biosynthesis of nanoparticles with the extracts of Z. xylopyrus prepared by three different methods of open heating, ultrasonication and reflux heating were studied as per the methodology described in “Effect of pH”. SPR spectra of the synthesized nanoparticles using the extracts prepared by three different methods and at different pH conditions are presented in Fig. 3a–c. At pH 3, a flat absorption spectrum was observed. No SPR peak was formed under acidic conditions hence terming it as unsuitable for promoting biosynthesis of silver nanoparticles while neutral and alkaline conditions demonstrated SPR peaks indicating that these conditions of pH are suitable for biosynthesis of Ag nanoparticles. Higher SPR peak intensities at alkaline condition, indicates increased number of smaller silver nanoparticles. The peak signifies uniformly shaped nanoparticles. A possible explanation for alkaline condition to be favorable for this would be that due to alkalinity, hydroxides get deposited on the silver nanoparticle. It may be hypothesized in the similar lines as that by Oza et al. (2012) in their explanation on biosynthesis of gold nanoparticles, that at alkaline pH both reducing as well as capping agents are efficiently reducing the particles and further encapping them at specific facets. This allows growth of spherical nanoparticles due to vulnerable deposition of silver atoms on all the facets forming thermodynamically favorable spherical nanoparticles. Due to very high proton concentration at acidic pH, all the functional groups responsible for biosynthesis of nanoparticles possess positive charge. Thus even if the nanoparticles are formed, they are not stable enough to prevent agglomeration. The reducing power of these functional groups at lower pH is less, but as the pH increases to alkalinity range, the reduction potentials of all these functional groups are enhanced, thus allowing the formation of thermodynamically favorable structures. Hence alkaline condition is favorable for the biosynthesis. Sathishkumar et al. (2009) in their studies on effect of pH on nanoparticle synthesis have also observed small and stable nanoparticles being formed at alkaline pH. According to them, Lower pH values promote the nucleation of the nanoparticles while higher pH caused electrostatic repulsion among them thereby leading to formation of smaller nanoparticles. In the present study, in all the three cases of utilizing the extracts prepared by different methods, it is observed that acidic condition suppressed the formation of silver nanoparticles and the basic condition enhanced the formation of silver nanoparticles.
According to Mie’s theory (1908), only a single SPR band expected in the absorption spectra of spherical nanoparticles, where as anisotropic particles could give rise to two or more SPR bands depending on the shape of the particles. The spherical nanoparticles, disks and triangular nanoparticles of silver show one, two and more peaks, respectively (Mukherjee et al. 2012). The single SPR band noticed in the UV–Visible spectroscopic studies in the current study also substantiates the spherical nature of silver nanoparticles.
Comparison of extraction methodology in terms of conversion of silver ions to silver nanoparticles
Conversion of silver nitrate to silver nanoparticles during biosynthesis with reaction mixture at optimum pH of 11 and at natural pH of the extract (without any pH adjustment), with all the three types of extracts was calculated. In these studies, silver nanoparticles were biosynthesized using 10 mM AgNO3 solution. From Fig. 4, it can be seen that with the extract prepared by reflux method higher conversion of silver ions to nanoparticles was achieved, followed by that with ultrasonication method and open heating. In reflux heating method, solvent loss by evaporation is minimal and the desired temperature is maintained throughout the reflux operation. This leads to effective extraction of different bioactive components of the bark like antioxidants, phenolics, etc., by the solvent. But in open heating extraction method, solvent loss may occur by evaporation and temperature is lower than the boiling temperature of the solvent throughout the extraction process, which leads to less efficient extraction of bioactive components. Ultrasonication method seems to be more efficient than open heating, as it accelerates the release of bioactive components into the solvent. Solvent extraction with reflux (Fig. 5) is the best method among the others studied. Biosynthesis at optimum pH condition of 11 has been proven to be favorable as compared to the natural pH conditions, as indicated by higher conversion to silver nanoparticles at pH of 11.
SEM and EDX analysis of the biosynthesized silver nanoparticle
Biosynthesized nanoparticles (under optimum conditions using 10 mM silver nitrate solution and alkaline initial pH) were found to be spherical in nature as shown in the SEM image (Fig. 6) and ranged from 60 to 70 nm in size. The EDX spectrum shown in Fig. 7 displays the presence of silver in the sample, further confirming the presence of silver nanoparticles and also the presence of carbon and oxygen suggesting that the silver nanoparticles must be capped by the organic components present in the plant extract. AgNPs are established as antimicrobial agents, while the presence of plant bioorganic capping material upon the AgNPs enable them to exhibit enhanced antibacterial activity (MubarakAli et al. 2011; Prabhu and Poulose 2012) and can be used as antioxidant agents (Niraimathi et al. 2013).
Conclusions
Zizyphus xylopyrus bark extracts can be efficiently used for the biosynthesis of silver nanoparticles. UV–Visible spectroscopy showed peaks in the range 413–420 nm confirming the formation of silver nanoparticles. Different factors affecting the synthesis of silver nanoparticles like methodology for the preparation of extract, concentration of silver nitrate solution used for biosynthesis and initial pH of the reaction mixture were studied. Silver nitrate solution of 10 mM concentration and alkaline initial pH of 11, which resulted in SPR peak of high intensity indicating enhanced synthesis of silver nanoparticles can be considered the optimum conditions for the biosynthesis. The extract prepared by reflux extraction method at optimum initial pH resulted in higher conversion of silver ions to silver nanoparticles as compared with those prepared by other extraction methods. SEM analysis showed that the biosynthesized nanoparticles are spherical in nature and ranged from 60 to 70 nm in size. EDX showed the presence of carbon and oxygen with Ag, suggesting that the silver nanoparticles must be capped by the organic components present in the plant extract for elucidating the bioconversion of the precursor salt into AgNPs (Patil et al. 2012b). This simple process for the biosynthesis of silver nanoparticles using aqueous extract of Z. xylopyrus, is a green technology with no use of hazardous and toxic solvents and chemicals and hence is environment friendly. The process has several advantages with reference to cost and compatibility for the bio-organic compound capped AgNPs to be used as antimicrobial agents, in water purification systems, for its application in medical and drug delivery, as well as for large scale commercial production.
References
Anarkali J, Raj DV, Rajathi K, Sridhar S (2010) Biological synthesis of silver nanoparticles by using Mollugo nudicaulis extract and their antibacterial activity. Arch Appl Sci Res 4:1436–1441
Ashmi M, Golap K, Madhuri S, Oza G, Sunil P (2012) Extracellular biosynthesis of gold nanoparticles using escherichia coli and deciphering the role of lactate-dehydrogenase using ldh knock out E. Coli. Adv Appl Sci Res 3:1405–1412
Bhainsa KC, D’Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Coll Surf B Biointerfaces 47:160–164
Chen D, Qiao X, Qiu X, Chen J (2009) Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J Mater Sci 44:1076–1081
Costa C, Conte A, Buonocore GG, Del Nobile MA (2011) Antimicrobial silver-montmorillonite nanoparticles to prolong the shelf life of fresh fruit salad. Int J Food Microbiol 148:164–167
Cruz D, Fale PL, Mourato A, Vaz PD, Serralheiro ML, Lino AR (2010) Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena). Coll Surf B Biointerface 81:67–73. doi:10.1016/j.colsurfb.2010.06.025. Epub 2010 Jul 4
Dauthal P, Mukhopadhyay M (2013) In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. J Nanopart Res 15:1366. doi:10.1007/s11051-012-1366-7
Dubey SP, Lahtinen M, Sillanpaa M (2010) Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugos. Process Biochem 45:1065–1071
Duncan TV (2011) Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Coll Interface Sci 363:1–24
Jain P, Pradeep T (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng 90:59–63
Jiale H, Guowu Z, Bingyun Z, Daohua S, Fenfen L, Yuan L, Huimei C, Zhouding Z, Yanmeig Z, Qingbiao L (2011) Biogenic silver nanoparticles by Cacumen platycladi extract: synthesis, formation mechanism, and antibacterial activity. Ind Eng Chem Res 50:9095–9106
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 79:594–598
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677
Kim BS, Lee SH, Salunke BK (2014) Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using magnolia kobus plant leaf extracts. Biotechnol Bioprocess Eng 19:169–174. doi:10.1007/s12257-013-0561-4
Kumar GG, Sangita MS, Bappadity M, Kiran M (2011) Phytochemical evaluvation of methanolic extract of Zizyphus xylopyrus. Int J Drug Discov Herb Res 1:231–233
Kumar R, Mohana Roopan S, Prabhakarn A, Khanna VG, Chakroborty S (2012) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc 90:173–176
Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, Alvarez PJ (2008) Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res 42:4591–4602
McFarland AD, Van Duyne RP (2003) Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett 3:1057–1062
Mervat F, Zayed Wael H, Eisa Shabaka AA (2012) Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 98:423–428
Mie G (1908) Beiträge zur optik trüber medien. Ann Phys 25:374–445
Moon KS, Dong H, Maric R, Pothukuchi S, Hunt A, Li Y, Wong CP (2005) Thermal behavior of silver nanoparticles for low-temperature interconnect applications. J Electron Mater 34:168–175
MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Coll Surf B 85:360–365
Mukherjee P, Senepati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2012) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chem Biochem 5:461–463
Niraimathi KL, Sudha V, Lavanya R, Brindha P (2013) Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Coll Surf B 102:288–291
Patil RS, Kokate MR, Kolekar SS (2012a) Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 91:234–238
Patil SV, Borase HP, Patil CD, Salunke BK (2012b) Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol 167:776–790. doi:10.1007/s12010-012-9710-z
Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 24:245705
Philip D (2011) Mangifera Indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 8:327–333
Philip D, Aswathy Unni C, Aromal S, Vidhu VK (2011) Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 78:899–904
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:1–10
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A (2011) Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Coll Surf B Biointerfaces 82:152–159
Renugadevi K, Venus AR (2012) Microwave irradiation assisted synthesis of silver nanoparticle using Azadirachta indica leaf extract as a reducing agent and invitro evaluation of its antibacterial and anticancer activity. Int J Nanomater Biostruct 2:5–10
Safekordi AA, Attar H, Ghorbani HR, Sorkhabadi S, Rezayat M (2011) Optimization of silver nanoparticles production by E.coli Bacterium (DH5 alpha) and the Study of Reaction Kinetics. Asian J Chem 23:5111–5118
Saima N, Bushra S, Muhammad S, Khalil R (2013) Alteration in antioxidant and antimicrobial attributes of leaves of Zizyphus species in response to maturation. J Med Plants Res 7:61–70
Sastry M, Patil V, Sainkar SR (1998) Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J Phys Chem B 102:1404–1410
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Coll Surf B Biointerfaces 73:332–338
Thirunavoukkarasu M, Balaji U, Behera S, Panda PK, Mishra BK (2013) Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochim Acta A Mol Biomol Spectrosc 116:424–427
Usha Rani P, Reddy R (2011) Green synthesis of silver-protein (core-shell) nanoparticles using Piper betle L. leaf extract and its ecotoxicological studies on Daphnia magna. Coll Surf A Physicochem Eng Asp 389:188–194
Vimal KS, Nagendra SC, Santram L, Singhai AK (2009) Antidepressent activity of Zizyphus xylopyrus. Int J Phytomed 1:12–17
Yoon WJ, Jung KY, Liu J, Duraisamy T, Revur R, Teixeira FL, Berger PR (2010) Plasmon-enhanced optical absorption and photocurrent in organic bulk heterojunction photovoltaic devices using self-assembled layer of silver nanoparticles. Sol Energy Mater Sol Cells 94:128–132
Zaki S, ElKady MF, Abd-El-Haleem D (2011) Determination of the effective origin source for nanosilver particles produced by Escherichia coli strain S78 and its application as antimicrobial agent. Mater Res Bull 46:1571–1576
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sumi Maria, B., Devadiga, A., Shetty Kodialbail, V. et al. Synthesis of silver nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl Nanosci 5, 755–762 (2015). https://doi.org/10.1007/s13204-014-0372-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0372-8