Abstract
Catalytic behavior of perchloric acid when supported to mesoporous silica SBA-15 (SBA-15/HClO4) was investigated as a heterogeneous Bronsted acid. Its reactivity and leaching possibility were studied in cascade ring opening-cyclocondensation sequence of diketene and alcohol with aldehyde in the presence of either of urea or ammonium acetate. Results showed that this catalyst can be highly recyclable for several cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Design and synthesis of mesoporous silica materials for catalytic aims are a highly demanding research area, nowadays [1,2,3]. High surface area, suitable pore channels compared to zeolites for small molecules, chemoselectivity over mesoporosity, feasibility in post-modification, and higher stability under harsh conditions are some aspects that can be pointed out why mesoporous silica materials are popular for catalytic tasks [1, 4,5,6,7]. Pore channel’s dimensional array (whether 2D or 3D) thickness of pore wall, synthesis procedure, surface area, stability over several reuses, and pore size distribution are some fateful factors in choosing a specific mesopore for catalyst design [8]. Among them, stability, easy synthetic procedure, and higher surface area are some reasons that have made SBA-15 as a most common mesoporous silica which is incorporated for catalyst designing [9].
Synthesis of heterogeneous Bronsted acid is a universal demand toward greening the chemical processes to eliminate or reduce the toxic and harmful chemicals release to environment [10,11,12].
So far, there have been many developments in post-modification strategies to convert SBA-15 to a mesoporous supported solid Bronsted acid. Organosiloxane precursors play a major role among the others. Sulfonic acid [13], carboxylic acid [14], and phosphonic acid [15]-based precursors are most common types for conversion of relatively neutral SBA-15 to Bronsted acid. However, they are generally time-consuming, complex, and expensive procedures which can be replaced by an easier and economical pathway. Herein, we have supported perchloric acid to SBA-15 through a proper method. Supporting perchloric acid onto silica gel is extensively studied as a catalyst in various organic transformations. Hence, in this paper, we supported perchloric acid onto SBA-15 to generate a new catalyst based on Bronsted acidic feature.
The use of SBA-15/HClO4 as a catalyst has been achieved in the catalysis of two series of multicomponent and cascade type syntheses of heterocycles including dihydropyrimidinones (DHPM) and dihydropyridines (DHP). These compounds exhibit a series of biological activities such as calcium channel blocking, antihypertensive agents, antiviral, anti-carcinogenic, antiinflammatory, antibacterial, and melanin concentrating hormone receptor antagonists [16,17,18,19].
In detail, we used SBA-15/HClO4 in catalytic ring-opening reaction of diketene in the presence of alcohols and aldehydes for the synthesis of DHPMs and DHPs’ heterocycles via final nucleophilic attack of urea and ammonium acetate, respectively (Fig. 1).
Experimental
Chemicals and apparatus
All reagents were obtained from Merck (Germany) and Fluka (Switzerland), and were used without further purification. Melting point was measured on an Electrothermal 9100 apparatus. Progress of reactions was monitored by thin-layer chromatography (TLC). 1H and 13C NMR spectra were measured (CDCl3) with a Bruker DRX-300 AVANCE spectrometer at 300 and 75 MHz, respectively. All yields were calculated based on the isolated amount of products.
Ordered mesoporous silica supported perchloric acid: synthesis of SBA-15/HClO4
For synthesis of SBA-15 [20], triblock copolymer ethylene oxide-propylene oxide-ethylene oxide (P123, 4.0 g) as a structure directing agent was dissolved in mixture solution containing deionized water (30 mL) and 120 g of 2 M HCl. Then, TEOS (9 g) was added to reaction mixture to stir for 24 h at 40 °C. Then, the resultant mixture was transferred into a Teflon-lined stainless steel autoclave and kept for 24 h at 100 °C to complete aging. After cooling down to room temperature, the product was filtered, washed with water, and dried overnight at 70 °C in air. Afterward, the obtained sample after aging was raised to 600 °C by ramp of 1 °C/min and kept at that temperature for 6 h to calcine and remove the template. Then, the calcined SBA-15 (1 g) was treated by perchloric acid (HClO4) in toluene (2 mmol) under reflux conditions for 24 h. Finally, to purify SBA-15/HClO4 and eliminate the unsupported HClO4 was then washed with distilled water for several times and dried at 55–60 °C. H+ titration analysis was achieved and the loaded amount of H+ onto SBA-15 was 1.7 mmol g−1. BET analysis showed a surface area of ca. 538 m2 g−1 for the obtained catalyst.
FT-IR spectra of SBA-15/HClO4 exhibit some characteristic peaks at 800 and ~ 1150 cm−1 which can be assigned to (Si–O–Si) bond vibrations, with a small band at ca. 950 cm−1 can be attributed to unfunctionalized silanol groups. Therefore, it can be judged that silanol groups are functionalized in post-modification step. –O–H of silanol groups can be visualized in the very broad IR absorption band in 3000–3700 cm−1 region (Fig. 2).
Figure 3 shows images of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) of the as-synthesized SBA-15/HClO4. The highly ordered mesoporous structure of SBA-15/HClO4 observed by small-angle XRD confirms the highly ordered and aligned mesochannels in TEM image (Fig. 3a, c). Diffraction peaks at the below 2° corresponding to the (1 0 0), (1 1 0), and (2 0 0) are characteristic from the XRD pattern of SBA-15 (Fig. 3c).
General procedure for the synthesis of DHPMs and DHPs under neat condition over SBA-15/HClO4
Equivalent amount of pure diketene, alcohol in the presence of an excess amount of equivalent, and aldehyde in SBA-15/HClO4 (10 mol%) for 10 min under reflux conditions at 100 °C, NH4OAc or urea was added, and then heated under neat and refluxing conditions under vigorous stirring, during an appropriate time (see Tables 1, 2). The reaction was cooled to room temperature and the solid was washed with dichloromethane. All the products are known, and they are previously reported in the literatures [21,22,23,24] and characterized by comparing physical data. In recycle procedure, the model reactions were done according to optimized conditions in 5 mmol of reaction scale.
Results and discussion
The use of SBA-15/HClO4 as a heterogeneous and recyclable solid Bronsted acid is designed in this paper and used as a promising catalyst for organic transformations. The reaction schemes are based on the use of diketene for in situ generation of β-ketoesters for using it in the four-component synthesis of dihydropyrimidinones and dihydropyridines (Scheme 1). Solid acid catalyst can be easily prepared from the readily available ingredients, perchloric acid, and silica mesostructure.
The reaction of diketene, arylaldehydes 1, and alcohols 2, with urea in the presence of a catalytic amount of SBA-15/HClO4 undergoes a Biginelli-type cyclocondensation reaction under reflux conditions which produces DHPM derivatives 3 in 86–93% yields (Table 1).
1,4-Dihydropyridine products 4 were generated with ammonium acetate in high yields. The results are summarized in Table 2. A possible explanation on the mechanism of these reactions is proposed in Scheme 2. The in situ generation of β-dicarbonyl 5 and unsaturated adduct 6 happens by addition of the alcohol and aldehyde, respectively, to diketene under acidic and reflux conditions which finally result in DHPMs and DHPs as Biginelli-type and Hantzsch-type conditions, respectively (Tables 1, 2).
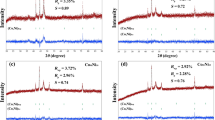
The possibility of reusing SBA-15/HClO4 catalyst in the syntheses of the 3h and 4g as model reactions was tested and studied. During this study, at the end of reaction completion (Tables 1, 2), the catalyst was easily separated from the product by centrifugation. As indicated in Fig. 4, recyclability through several consecutive runs is preserved which shows that the leaching of perchlorate from modified surface is insignificant. It also shows that there is a powerful interaction between silica and perchlorate. This observation is in agreement with other previous reports about silica–perchlorate interaction [25,26,27,28].
As shown in Scheme 2, when the four-component reactions were performed in the presence of t-butyl alcohol both as a solvent and one of the components in the reaction, results showed a mixture of product and by-products. In addition, in the case of isopropyl alcohol, there was a similar result like t-butyl alcohol.
Conclusion
In summary, non-covalent functionalization of perchloric acid onto the ordered mesoporous silica SBA-15/HClO4 as a simply attainable heterogeneous, green, and reusable Bronsted acid catalyst for cascade ring-opening and cyclization towards heterocycles through diketene compound is successfully achieved. By this method, DHPMs and DHPs are accessible under ambient conditions, that this solid acidic catalytic procedure based on neat conditions, is truly recoverable compared to irrecoverable homogenous methods. Such an approach avoids the use of expensive catalyst and solvents or other additives. This rapid, green, and reusable catalyst in four-component reactions was interesting alternative to the other either of homogeneous or solid Bronsted acid catalysts.
References
Sun, L.-B., Liu, X.-Q., Zhou, H.-C.: Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 44, 5092–5147 (2015)
Rostamnia, S., Doustkhah, E.: Nanoporous silica-supported organocatalyst: a heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 4, 28238–28248 (2014)
Doustkhah, E., Rostamnia, S., Imura, M., Ide, Y., Mohammadi, S., Hyland, C.J.T., You, J., Tsunoji, N., Zeynizadeh, B., Yamauchi, Y.: Thiourea bridged periodic mesoporous organosilica with ultra-small Pd nanoparticles for coupling reactions. RSC Adv. 7, 56306–56310 (2017)
Hosseini, H.G., Doustkhah, E., Kirillova, M.V., Rostamnia, S., Mahmoudi, G., Kirillov, A.M.: Combining ethylenediamine and ionic liquid functionalities within SBA-15: a promising catalytic pair for tandem Cu–AAC reaction. Appl. Catal. 548, 96–102 (2017)
Doustkhah, E., Rostamnia, S.: Chapter 9—palladium complexes and nanoparticles encapsulated by functionalized mesoporous silica materials: a promising hybrid catalyst in organic transformations A2—Sadjadi, Samahe. In: Encapsulated Catalysts, pp. 279–307. Academic Press, Cambridge (2017).
Doustkhah, E., Rostamnia, S.: Single site supported N-sulfonic acid and N-sulfamate onto SBA-15 for green and sustainable oxidation of sulfides. Mater. Chem. Phys. 177, 229–235 (2016)
Rostamnia, S., Doustkhah, E., Zeynizadeh, B.: Cationic modification of SBA-15 pore walls for Pd supporting: Pd@SBA-15/ILDABCO as a catalyst for Suzuki coupling in water medium. Microporous Mesoporous Mater. 222, 87–93 (2016)
Pal, N., Bhaumik, A.: Mesoporous materials: versatile supports in heterogeneous catalysis for liquid phase catalytic transformations. RSC Adv. 5, 24363–24391 (2015)
Vavsari, V.F., Ziarani, G.M., Badiei, A.: The role of SBA-15 in drug delivery. RSC Adv. 5, 91686–91707 (2015)
Motokura, K., Nakagiri, N., Mori, K., Mizugaki, T., Ebitani, K., Jitsukawa, K., Kaneda, K.: Efficient C − N bond formations catalyzed by a proton-exchanged montmorillonite as a heterogeneous brønsted acid. Org. Lett. 8, 4617–4620 (2006)
Zhang, L., Cui, Y., Zhang, C., Wang, L., Wan, H., Guan, G.: Biodiesel production by esterification of oleic acid over brønsted acidic ionic liquid supported onto Fe-incorporated SBA-15. Ind. Eng. Chem. Res. 51, 16590–16596 (2012)
Shirini, F., Atghia, S.V., Mamaghani, M.: Sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite as an efficient, eco-benign, and water-tolerant nanoreactor for chemoselective oxathioacetalization of aldehydes. Int. Nano Lett. 3, 3 (2013)
Rostamnia, S., Doustkhah, E.: Increased SBA-15-SO3H catalytic activity through hydrophilic/hydrophobic fluoroalkyl-chained alcohols (RFOH/SBA-15–Pr-SO3H). Synlett 26, 1345–1347 (2015)
Nandi, M., Mondal, J., Sarkar, K., Yamauchi, Y., Bhaumik, A.: Highly ordered acid functionalized SBA-15: a novel organocatalyst for the preparation of xanthenes. Chem. Commun. 47, 6677–6679 (2011)
Corriu, R.J.P., Datas, L., Guari, Y., Mehdi, A., Reye, C., Thieuleux, C.: Ordered SBA-15 mesoporous silica containing phosphonic acid groups prepared by a direct synthetic approach. Chem. Commun. 8, 763–764 (2001)
Nagarajaiah, H., Mukhopadhyay, A., Moorthy, J.N.: Biginelli reaction: an overview. Tetrahedron Lett. 57, 5135–5149 (2016)
Wani, M.Y., Ahmad, A., Kumar, S., Sobral, A.J.F.N.: Flucytosine analogues obtained through Biginelli reaction as efficient combinative antifungal agents. Microb. Pathog. 105, 57–62 (2017)
Maharramov, A.M., Ramazanov, M.A., Guliyeva, G.A., Huseynzada, A.E., Hasanova, U.A., Shikhaliyev, N.G., Eyvazova, G.M., Hajiyeva, S.F., Mamedov, I.G., Aghayev, M.M.: Synthesis, investigation of the new derivatives of dihydropyrimidines and determination of their biological activity. J. Mol. Struct. 1141, 39–43 (2017)
Ahmad, S., Iftikhar, F., Ullah, F., Sadiq, A., Rashid, U.: Rational design and synthesis of dihydropyrimidine based dual binding site acetylcholinesterase inhibitors. Bioorg. Chem. 69, 91–101 (2016)
Doustkhah, E., Rostamnia, S., Hossieni, H.G., Luque, R.: Covalently bonded PIDA on SBA-15 as robust Pd support: water-tolerant designed catalysts for aqueous suzuki couplings. ChemistrySelect 2, 329–334 (2017)
Ma, Y., Qian, C., Wang, L., Yang, M.: Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 65, 3864–3868 (2000)
Sadanandam, Y., Shetty, M., Diwan, P.: Synthesis and biological evaluation of new 3, 4-dihydro-6-methyl-5-N-methyl-carbamoyl-4-(substituted phenyl)-2 (1H) pyrimidinones and pyrimidinethiones. Eur. J. Med. Chem. 27, 87–92 (1992)
Shaabani, A., Seyyedhamzeh, M., Maleki, A., Rezazadeh, F., Behnam, M.: New one-pot four-component synthesis of disubstituted pyrido [2, 3-d] pyrimidine-6-carboxamide derivatives. J. Comb. Chem. 11, 375–377 (2009)
Janis, R.A., Triggle, D.: New developments in calcium ion channel antagonists. J. Med. Chem. 26, 775–785 (1983)
Farrokhzadeh, A., Modarresi-Alam, A.R.: Complete doping in solid-state by silica-supported perchloric acid as dopant solid acid: Synthesis and characterization of the novel chiral composite of poly [(±)-2-(sec-butyl) aniline]. J. Solid State Chem. 237, 258–268 (2016)
Ansari, M.I., Hussain, M.K., Yadav, N., Gupta, P.K., Hajela, K.: Silica supported perchloric acid catalyzed rapid N-formylation under solvent-free conditions. Tetrahedron Lett. 53, 2063–2065 (2012)
Das, B., Reddy, P.R., Sudhakar, C., Lingaiah, M.: Efficient dehydrative C-N bond formation using alcohols and amides in the presence of silica supported perchloric acid as a heterogeneous catalyst. Tetrahedron Lett. 52, 3521–3522 (2011)
Narasimhulu, M., Reddy, T.S., Mahesh, K.C., Prabhakar, P., Rao, C.B., Venkateswarlu, Y.: Silica supported perchloric acid: a mild and highly efficient heterogeneous catalyst for the synthesis of poly-substituted quinolines via Friedländer hetero-annulation. J. Mol. Catal. A 266, 114–117 (2007)
Acknowledgements
We are thankful from Payame-Noor University for financial supports on accomplishment of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Baghban, A., Doustkhah, E. & Rostamnia, S. Catalytic behavior of perchloric acid on silica mesoporous SBA-15 as a green heterogeneous Bronsted acid in heterocyclic multicomponent reactions. Int Nano Lett 8, 41–47 (2018). https://doi.org/10.1007/s40089-018-0231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-018-0231-9