Abstract
Iron(III) supported on nano silica as a new catalyst has been synthesized. Structural properties of this complex have been studied by TEM, SEM and EDX. The average crystalline size of Iron(III) supported on nano silica is 30–50 nm. Catalytic activity of this catalyst has been investigated by synthesis of benzimidazoles from 1, 2-diaminobenzene and aromatic aldehydes, and also the other variables investigated such as the amount of catalyst, reaction temperature and the effect of various solvents are also studied. The present procedure offers several advantages such as short reaction time, simple workup, recovery and reusability of the catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
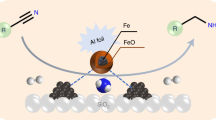
Solid-supported reagents are completely acid catalysts that have become known over the last decades. The activity and selectivity of a reagent dispersed on the surface of a support are reformed, as the effective surface field of the reagent is improved multifold and thus they are more effective than the separate reagents [3]. Amongst various solid supports, Nano silica is usually preferred, since it exhibits many useful properties such as high surface area, excellent stability (thermal and chemical), and good availability. Besides, organic groups can be robustly anchored to the surface to provide catalytic centers [4, 6, 19]. Benzimidazole nucleus is an important intermediate with its good biological and pharmacological properties in the organic synthesis [1]. It exhibits substantial activity against several viruses such as HIV [15] and herpes (HSV-1) [12]. Methods of synthesis benzimidazole include the reaction between o-aryldiamines and aldehyde in refluxing nitrobenzene [17, 20], the condensation of o-aryldiamines with carboxylic acids or their derivatives in the presence of robust acids such as polyphosphoric acid [14] or inorganic acids [8]. Direct concentration of o-aryldiamines and aldehydes is not a good synthetic reaction, as it is well known to yield a complex combination, being 1,2-disubstituted benzimidazoles [5]. In this case, the addition of transition metal, namely mercury oxide [9] or lead tetraacetate [16], allows a partial selective synthesis of benzimidazoles. In current years, solvent-free synthesis of benzimidazoles have been reported using Yb (OTf)3 [18], KSF clay [10], PPA [11], solid-support [13], and iodine mediated in aqueous condition [16], Indion 190 resin [2]. Unfortuna tely, many of these processes suffer some limitations, such as drastic reaction conditions, low yields, tedious work-up processes and co-occurrence of several side reactions. Following our work [7], in this paper, we reported a nano heterogeneous catalyst with supported Fe(III) on the Nano SiO2 and morphological of the catalyst was investigated by FTIR and SEM. In addition, this catalyst is an efficient method to prepare benzimidazoles derivatives and recyclable catalyst under solvent-free conditions (Scheme 1). The merit of this methodology is that it is simple, fast, mild and efficient.
Experimental
All reagents and solvents were commercially available and were used as such. Silicon Oxide (SiO2, 99.5 %, 15 nm) was purchased from Nanostructured and Amorphous Materials, Inc. The morphology of catalysts and their precursors was observed by means of a Philips XL30 scanning electron microscopy (SEM). Melting points were determined using Barnstead—Electrothermal 9300 Melting Point. The products were isolated and characterized by physical and spectral data. 1H NMR and 13CNMR spectra were recorded on Bruker Avance-200 MHz spectrometers in the presence of tetramethylsilane as internal standard.
General procedure for the preparation of nano silica supported ferric chloride
To achieve a homogeneous adsorption, in a 25 mL flask, nano silica gel (0.1 g) and FeCl3·6H2O (0.004 g) (4 % of the weight of nano-SiO2) were vigorously stirred by magnetic stirrer under solvent-free conditions at 75 °C for 24 h. A yellow powder was obtained. This powder was heated for 1 h at 100 °C to give a brownish powder, (iron/nano silica.reagent).
General procedure for the preparation of benzimidazole
To a mixture of o-phenylenediamine (1 mmol) and arylaldehydes (1 mmol) aq., 30 % H2O2 (1 mmol) and SiO2–FeCl3 (0.05 g) were added and the mixture was heated at 75 °C for 10 min. The progress of the reaction was monitored by TLC (eluent: n-hexane–EtOAc, 7:3). When the starting materials completely disappeared, the mixture was cooled at room temperature, and then the solid was dissolved in CH2Cl2 (10 mL). The catalyst was separated and the organic layer was washed with water (2 × 10 mL) and dried under MgSO4. The filtrate was evaporated and the corresponding benzimidazole was obtained as the only product after recrystallization in aq. Ethanol (25 %).
Results and discussions
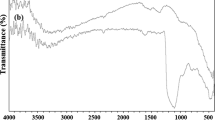
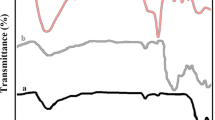
In the first step, iron/silica nano composite was prepared by mixing nano silica gel (0.1 g) and FeCl3·6H2O (0.004 g). Then, it was vigorously stirred by magnetic stirrer under solvent-free conditions at room temperature for 24 h to achieve a homogeneous adsorption. However, to do so, various parameters were investigated such as the presentation of iron ratios to nano-SiO2 and temperature of mixing for preparing nano iron/silica in the synthesis of 2-(4-chlorophenyl)-1H-benzimidazole by condensing o-phenylenediamine with 4-chlorobenzaldehyde. So, the best condition for the synthesis of 2-(4-chlorophenyl)-1H-benzimidazole is 0.05 g of FeCl3·6H2O (4 % of the nano-SiO2 weight) at 75 °C (Fig. 1a, b). The nano composite catalytic behavior was compared with several types of catalysts in the reaction to benzimidazole (Table 1). In the absence of a catalyst, the reaction did not progress at all. Notably, nano iron/silica composite shows an activity higher than those reported in heterogeneous. We believe that nano silica surface chemistry plays an important role in this reaction. In next study, the nano composite catalyst was characterized through TEM, SEM, EDX, and line scan. The morphology and particle size of these iron/silica nanocatalyst were investigated by TEM, as shown in Fig. 2. The shape of NPs is spherical with average particle size of 30–50 nm. The characterization of catalyst synthesized from the iron/silica (4 % of the nano-SiO2 weight) was also carried out using scanning electron microscopy. SEM-EDX the electron micrograph was obtained from powder specimens of these materials, and SEM-EDX is used to obtain information about their microstructural and metal dispersion properties, so the morphology of catalyst with SEM was shown in Fig. 3. The weight percentages of the iron/silica nano composite over the surface of catalyst were determined by EDX; the Si and Fe weight percentages are 57.17 and 2.103, respectively. In particular, Si and Fe are observed to be well on the surface of catalyst (Fig. 4a). Figure 4b shows the line scan obtained in an iron/silica nano composite catalyst. The line scan of the catalyst is placed at around 60 µm from the beginning of the line scan, so it was shown that Fe is still clearly visible. To find optimal amount of nano composite, the reaction was carried out by varying the amount of the catalyst. The catalytic conversion was increased by increasing the weight of catalyst up to 10–50 mg and then it became constant with further increase in the weight. These results indicate that for this conversion a different scale of Lewis site is required. For more investigation, we have screened the effects of different solvents with varying polarity and protic nature. It was observed that solvent-free condition is proved to be the best choice for this reaction over any methanol, ethanol and organic solvents such as acetonitrile, CH2Cl2 and DMF. Also, it was observed that the good results were not obtained in operation in nitrogen atmosphere conditions. The results are presented in Table 2. The recovered catalyst from the experiment was washed by acetone (3 × 5 mL). Then, it was dried in an oven at 100 °C and used in the synthesis of 2-phenyl-1H-benzo[d]imidazole. Then, the catalyst was recycled for five times (Table 3). The study was then extended to prepare various benzaldehydes using silica (NPs) supported Fe(III) in high yields. The reactions were carried out in solvent-free conditions and at 75 °C. The results are listed in Table 4.
Conclusion
In conclusion, it was demonstrated that a novel readily available economic silica (NPs) supported Fe(III) has been prepared. This catalyst could behave as a recyclable and heterogeneous solid acid for the synthesis of various substituted benzimidazoles. The merit of this methodology is that it is simple, fast, mild and efficient.
References
Aiello, S., Wells, G., Stone, E.L., Kadri, H., Bazzi, R., Bell, D.R., Westwell, A.D.: Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3, 4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648)(1). J. Med. Chem. 51(16), 5135–5139 (2008)
Padalkar, V.S, Gupta, V.D, Phatangare, K.R, Patil, V.S, Umape, P.G, Sekar, N.: Indion 190 resin: efficient, environmentally friendly, and reusable catalyst for synthesis of benzimidazoles, benzoxazoles, and benzothiazoles. Green. Chem. Lett. Rev. 5(2), 139–145 (2012)
Corma, A., Garcia, H.: Silica-bound homogenous catalysts as recoverable and reusable catalysts in organic synthesis. Adv. Synth. Catal. 348(12–13), 1391–1412 (2006)
De Vos, D.E., Dams, M., Sels, B.F., Jacobs, P.A.: Ordered mesoporous and microporous molecular sieves functionalized with transition metal complexes as catalysts for selective organic transformations. Chem. Rev. 102(10), 3615–3640 (2002)
Smith, J.G., Ho, I.: Organic redox reactions during the interaction of o-phenylenediamine with benzaldehyde. Tetrahedron Lett. 12(38), 3541–3544 (1971)
Garg, B., Sharma, R., Bhojak, N., Mittal, S.: Chelating resins and their applications in the analysis of trace metal ions. Microchem. J. 61(2), 94–114 (1999)
Ghodrati, K., Farrokhi, A., Karami, C., Hamidi, Z.: Nano silica sulfuric acid an efficient and recoverable heterogeneous catalyst for the preparation of amidoalkyl naphthols under solvent-free conditions. Synth. React. Inorg Metal Organic Nano Metal Chem. (just-accepted) (2013)
Grimmett, M.: Imidazoles and their benzo derivatives. Compr. Heterocycl. Chem. 5, 457 (1984)
Jakobson, P., Jannicke, M., Meyer, F.: Zirconyl (IV) chloride-promoted synthesis of benzimidazole derivatives. Ber 29, 2682 (1896)
Loupy, A., Petit, A., Hamelin, J., Texier-Boullet, F., Jacquault, P., Mathe, D.: New solvent-free organic synthesis using focused microwaves. Synthesis (9), 1213–1234 (1998)
Lu, J., Yang, B., Bai, Y.: Microwave irradiation synthesis of 2-substituted benzimidazoles using PPA as a catalyst under solvent-free conditions. Synth. Commun. 32(24), 3703–3709 (2002)
Migawa, M.T., Girardet, J.-L., Walker, J.A., Koszalka, G.W., Chamberlain, S.D., Drach, J.C., Townsend, L.B.: Design, synthesis, and antiviral activity of α-nucleosides: d-and l-isomers of lyxofuranosyl-and (5-deoxylyxofuranosyl) benzimidazoles. J. Med. Chem. 41(8), 1242–1251 (1998)
Perreux, L., Loupy, A.: A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 57(45), 9199–9223 (2001)
Preston, P.N.: The Chemistry of Heterocyclic Compounds, Benzimdazoles and Cogeneric Tricyclic Compounds, vol. 40. Wiley, New York (2009)
Roth, T., Morningstar, M.L., Boyer, P.L., Hughes, S.H., Buckheit, R.W., Michejda, C.J.: Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J. Med. Chem. 40(26), 4199–4207 (1997)
Sun, P., Hu, Z.: The convenient synthesis of benzimidazole derivatives catalyzed by I2 in aqueous media. J. Heterocycl. Chem. 43(3), 773–775 (2006)
Sun, Q., Yan, B.: Single bead IR monitoring of a novel benzimidazole synthesis. Bioorg. Med. Chem. Lett. 8(4), 361–364 (1998)
Wang, L., Sheng, J., Tian, H., Qian, C.: An efficient procedure for the synthesis of benzimidazole derivatives using Yb (OTf) 3 as catalyst under solvent-free conditions. Synth. Commun. 34(23), 4265–4272 (2004)
Wight, A., Davis, M.: Design and preparation of organic-inorganic hybrid catalysts. Chem. Rev. 102(10), 3589–3614 (2002)
Yadagiri, B., Lown, J.W.: Convenient routes to substituted benzimidazoles and imidazolo [4, 5-b] pyridines using nitrobenzene as oxidant. Synth. Commun. 20(7), 955–963 (1990)
Acknowledgments
Financial support from the Islamic Azad University of Kermanshah is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taher, M.A., Karami, C., Arabi, M.S. et al. Efficient FeCl3/SiO2 as heterogeneous nanocatalysis for the synthesis of benzimidazoles under mild conditions. Int Nano Lett 6, 85–90 (2016). https://doi.org/10.1007/s40089-015-0167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0167-2