Abstract
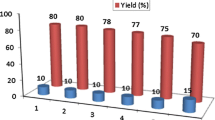
Efficient, green, simple and environmentally friendly approach for the straightforward reductive coupling of nitroarenes to the corresponding azoxyarenes has been developed in the presence of Fe3O4@SiO2@Co–Zr–Sb as a novel recyclable nanocatalyst. The Co–Zr–Sb trimetallic nanoparticles immobilized on silica-layered magnetite have been prepared by the co-precipitation method. The mesoporous catalyst has been characterized by FT-IR, SEM, EDX, VSM, TEM and XRD analyses. The chemoselective hydrogenation of nitrobenzenes was carried out successfully in refluxing water to afford the corresponding azoxybenzenes within 2–10 min in good to high yields. The reusability of the heterogeneous nanocatalyst has also been studied using the FT-IR and SEM analyses. The catalyst was utilized four times in sequential runs without significant loss of activity. The current research includes remarkable advantages of short reaction times, absence of hazardous organic solvents, mild reaction conditions, high yields, using water as a green solvent and the ability to utilize the recyclable nanomagnetic catalyst.













Similar content being viewed by others
References
M.I. Qadir, J.D. Scholten, J. Dupont, Catal. Sci. Technol. 5, 1459 (2015)
Y.F. Chen, J. Chen, L.J. Lin, G.J. Chuang, J. Org. Chem. 82, 11626 (2017)
A. Rezaeifard, M. Jafarpour, M.A. Naseri, R. Shariati, Dyes Pigments 76, 840 (2008)
J.M. Huang, J.F. Kuo, C.Y. Chen, J. Appl. Polym. Sci. 55, 1217 (1995)
D. Campbell, L.R. Dix, P. Rostron, Dyes Pigments 29, 77 (1995)
M. Gamberini, M.R. Cidade, L.A. Valotta, M.C. Armelin, L.C. Leite, Carcinogenesis 19, 147 (1998)
A.B.E. Vix, P. Muller-Buschbaum, W. Stocker, M. Stamm, J.P. Rabe, Langmuir 16, 10456 (2000)
C.L. Folcia, I. Alonso, J. Ortega, J. Etxebarria, I. Pintre, M.B. Ros, Chem. Mater. 18, 4617 (2006)
S. Ghosh, S.S. Acharyya, T. Sasaki, R. Bal, Green Chem. 17, 1867 (2015)
S.S. Acharyya, S. Ghosh, R. Bal, ACS Sustain. Chem. Eng. 2, 584 (2014)
C.F. Chang, S.T. Liu, J. Mol. Catal. A: Chem. 299, 121 (2009)
H.J. Shine, H.E. Mallory, J. Org. Chem. 27, 2390 (1962)
C.E. Weill, G.S. Panson, J. Org. Chem. 21, 803 (1956)
Y. Liu, B. Liu, A. Guo, Z. Dong, S. Jin, Y. Lu, Molecules 16, 3563 (2011)
Z. Hou, Y. Fujiwara, H. Taniguchi, J. Org. Chem. 53, 3118 (1988)
A. McKillop, R.A. Raphael, E.C. Taylor, J. Org. Chem. 35, 1670 (1970)
Y. Lu, J. Liu, G. Diffee, D. Liu, B. Liu, Tetrahedron Lett. 47, 4597 (2006)
J.R. Hwu, A.R. Das, C.W. Yang, J.J. Huang, M.H. Hsu, Org. Lett. 7, 3211 (2005)
N. Sakai, K. Fujii, S. Nabeshima, R. Ikeda, T. Konakahara, Chem. Commun. 46, 3173 (2010)
J.R. Hwu, C.S. Yau, S.C. Tsay, T.I. Ho, Tetrahedron Lett. 38, 9001 (1997)
A.R. Becker, L.A. Sternson, J. Org. Chem. 45, 1708 (1980)
B. Zhou, J. Song, T. Wu, H. Liu, C. Xie, G. Yang, B. Han, Green Chem. 18, 3852 (2016)
J.A. Hrabie, L.K. Keefer, Chem. Rev. 102, 1135 (2002)
M. Nakata, S. Kawazoe, T. Tamai, K. Tatsuta, H. Ishiwata, Y. Takahashi, Y. Okuno, T. Deushi, Tetrahedron Lett. 34, 6095 (1993)
B.T. Newbold, J. Org. Chem. 27, 3919 (1962)
M. Boudart, Chem. Rev. 95, 661 (1995)
M. Heitbaum, F. Glorius, I. Escher, Angew. Chem. Int. Ed. 45, 4732 (2006)
H. Hattori, Chem. Rev. 95, 537 (1995)
N. Mizuno, M. Misono, Chem. Rev. 98, 199 (1998)
M.R. Othman, Z. Helwani, Martunus, W.J.N. Fernando, Appl. Organomet. Chem. 23, 335 (2009)
A. Corma, H. Garcia, F.X. Llabres i Xamena, Chem. Rev. 110, 4606 (2010)
M. Gilanizadeh, B. Zeynizadeh, New J. Chem. 42, 8553 (2018)
M. Gilanizadeh, B. Zeynizadeh, Res. Chem. Intermed. 44, 6053 (2018)
A. Baghban, E. Doustkhah, S. Rostamnia, Int. Nano Lett. 8, 41 (2018)
M. Gilanizadeh, B. Zeynizadeh, J. Iran. Chem. Soc. 15, 2821 (2018)
B. Zeynizadeh, E. Gholamiyan, M. Gilanizadeh, Curr. Chem. Lett. 7, 121 (2018)
M. Gilanizadeh, B. Zeynizadeh, E. Gholamiyan, Iran. J. Sci. Technol. Trans. A: Sci. 43, 819 (2019)
B. Zeynizadeh, M. Gilanizadeh, New J. Chem. 43, 18794 (2019)
M. Gilanizadeh, B. Zeynizadeh, Polycycl. Aromat. Compd. (2019). (in press)
M. Gilanizadeh, B. Zeynizadeh, Res. Chem. Intermed. 45, 2811 (2019)
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
R.G. Chaudhuri, S. Paria, Chem. Rev. 112, 2373 (2012)
F. Niu, L. Zhang, S.Z. Luo, W.G. Song, Chem. Commun. 46, 1109 (2010)
R. Cano, D.J. Ramon, M.J. Yus, Org. Chem. 75, 3458 (2010)
T. Poursaberi, V. Akbar, S.M.R. Shoja, Iran. J. Chem. Chem. Eng. 34, 41 (2015)
M. Ma, J. Xie, Y. Zhang, Z. Chen, N. Gu, Mater. Lett. 105, 36 (2013)
M. Kotani, T. Koike, K. Yamaguchi, N. Mizuno, Green Chem. 8, 735 (2006)
K.V.S. Ranganath, J. Kloesges, A.H. Schafer, F. Glorius, Angew. Chem. Int. Ed. 49, 7786 (2010)
H. Cai, K. Li, M. Shen, S. Wen, Y. Luo, C. Peng, G. Zhang, X. Shi, J. Mater. Chem. 22, 15110 (2012)
B. Zeynizadeh, F. Faraji, RSC Adv. 9, 13112 (2019)
Z. Chen, Y. Qiu, X. Wu, Y. Ni, L. Shen, J. Wu, S. Jiang, Tetrahedron Lett. 59, 1382 (2018)
J.H. Kim, J.H. Park, Y.K. Chung, K.H. Park, Adv. Synth. Catal. 354, 2412 (2012)
G. Sharma, D. Kumar, A. Kumar, A.H. Al-Muhtaseb, D. Pathania, M. Naushad, G.T. Mola, Mater. Sci. Eng. C 71, 1216 (2017)
X. Liu, Z. Ma, J. Xing, H. Liu, J. Magn. Magn. Mater. 270, 1 (2004)
Y. Zhang, G.M. Zeng, L. Tang, D.L. Huang, X.Y. Jiang, Y.N. Chen, Biosens. Bioelectron. 22, 2121 (2007)
Acknowledgements
The authors gratefully acknowledge the financial support of this work by the research council of Urmia University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeynizadeh, B., Gilanizadeh, M. Green and highly efficient approach for the reductive coupling of nitroarenes to azoxyarenes using the new mesoporous Fe3O4@SiO2@Co–Zr–Sb catalyst. Res Chem Intermed 46, 2969–2984 (2020). https://doi.org/10.1007/s11164-020-04126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04126-7