Abstract
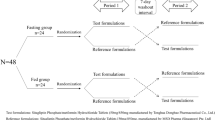
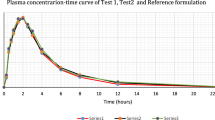
The aim of this study is to assess the quality of Telmi plus V® tablet (test formulation) by comparing its pharmacokinetic parameters with Micardis plus® tablet (reference formulation) in forty healthy Korean male volunteers. Seven subjects were withdrawn during the periods. This study was performed under fasted condition with a randomized, single-dose, 2-period crossover design. The dissolution studies of both formulations were conducted using USP apparatus 2 at 75 rpm with 1000 mL of phosphate buffer solution (pH 7.5) at 37 ± 0.5 °C. Forty healthy Korean male volunteers were enrolled in this study with 19–34 years (24.7 ± 3.9), height 164–184 cm (174.9 ± 5.3) and weight 52–85 kg (71.2 ± 9.0). The results of telmisartan were as follows: the mean AUC0−∞ of reference tablet and test tablet was 3290 ± 2270 and 3080 ± 2010 ng h/mL, respectively; the mean Cmax was 674 ± 366 and 612 ± 252 ng/mL, respectively; the mean Tmax was 0.92 ± 0.44 and 0.99 ± 0.46 h, respectively. The results of HCTZ were as follows: the mean AUC0−∞ of reference tablet and test tablet was 525 ± 94.3 and 536 ± 96.8 ng h/mL, respectively; the mean Cmax was 90.8 ± 25.4 and 86.2 ± 21.4 ng/mL, respectively; the mean Tmax was 1.58 ± 0.60 and 1.78 ± 0.66 h, respectively. The 90 % confidence intervals for geometric mean ratios of test to reference formulation of AUC0−t and Cmax were 0.9132–1.0441 and 0.8579–1.1048 for telmisartan, and 0.9737–1.0713 and 0.8801–1.0326 for HCTZ, respectively. The results of this study in healthy Korean male volunteers showed that the test and reference formulations of 80/12.5 mg telmisartan/hydrochlorothiazide met the MFDA regulatory criteria for bioequivalence. Both formulations were well tolerated, with no serious adverse events reported.


Similar content being viewed by others
References
Beermann B, Groschinsky-Grind M, Rosén A (1976) Absorption, metabolism, and excretion of hydrochlorothiazide. Clin Pharmacol Ther 19:531–537
Devineni D, Vaccaro N, Polidori D, Rusch S, Wajs E (2014) Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin Ther 36:698–710
FDA Guidance for industry (1997) Dissolution testing of immediate release solid oral dosage forms. Food and Drug Administration, Center for Drug Evaluation and Research, Rockville
Fenton C, Keating GM, Scott LJ (2003) Telmisartan/hydrochlorothiazide: in the treatment of essential hypertension. Drugs 63:2013–2026
Jordö L, Johnsson G, Lundborg P, Persson BA, Regärdh CG, Rönn O (1979) Bioavailability and disposition of metoprolol and hydrochlorothiazide combined in one tablet and of separate doses of hydrochlorothiazide. Br J Clin Pharmacol 7:563–567
Kim YH, Choi KS, Lee KH, Park JS (2012) Preparation and characterization of isosorbide 5-mononitrate extended-release tablets. J Pharm Investig 42:309–313
Kim JE, Ki MH, Yoon IS, Cho HJ, Kim RS, Kim GT, Kim DD (2014) Pharmacokinetic properties and bioequivalence of 2 formulations of valsartan 160-mg tablets: a randomized, single-dose, 2-period crossover study in healthy Korean male volunteers. Clin Ther 36:273–279
Lee YJ, Choi JH, Song SH, Seo CH, Kim DS, Park IS, Choi KH, Na HK, Chung SJ, Lee MH, Shim CK (1998) Development of K-BE test, a computer program for the analysis of bioequivalence. J Korean Pharm Sci 28:223–229
Lee YJ, Kim YG, Lee MG, Chung SJ, Lee MH, Shim CK (2000) Analysis of bioequivalence study log-transformed model. Yakhakhoeji 44:308–314
Lee YJ, Oh JH, Kim HG, Yi HJ, Shin YJ, Kim YG, Kim SN (2009) One-step sample size determination for 2 × 2 Bioequivalence study. J Korean Pharm Sci 39:217–219
Sajjan SS, Santoshkumar M, Sanjeevkumar S, Karegoudar TB (2013) Binding affinity of amlodipine, atorvastatin and telmisartan drugs to purified bacterial melanin pigment: a kinetic study. J Pharm Investig 43:267–278
Stangier J, Su CA, Roth W (2000a) Pharmacokinetics of Orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res 28:149–167
Stangier J, Schmid J, Türck D, Switek H, Verhagen A, Peeters PA, van Marle SP, Tamminga WJ, Sollie FA, Jonkman JH (2000b) Absorption, metabolism, and excretion of intravenously and orally administered [14C] telmisartan in healthy volunteers. J Clin Pharmacol 40:1312–1322
Takka S, Sakr A, Goldberg A (2003) Development and validation of an in vitro-in vivo correlation for buspirone hydrochloride extended release tablets. J Control Release 88:147–157
The Ministry of Food and Drug Safety (MFDA) (2012) Guideline for management of bioequivalence test (MFDA Notification No. 2012-103). September 2012
Thomas F, Gottfried S (2012) Bilayer pharmaceutical tablet comprising telmisartan and a diuretic. EP Patent 2260833
Wienen W, Entzeroth M, van Meel JCA, Stangier J, Busch U, Ebner T, Schmid J, Lehmann H, Matzek K, Kempthorne-rawson J, Gladigau V, Hauel NH (2000) A review on telmisartan: a novel, long-acting angiotensin II receptor antagonist. Cardiovasc Drug Rev 18:127–154
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects (2008) As amended by the 59th WMA General Assembly, Seoul, Oct 2008
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (K. M. Kim, W. -S. Hwang, J. H. Jung, B. Kim, G. T. Kim) declare that they have no confolict of interest. The authors are grateful to CMC R&D center, LG Life Sciences, Ltd. for supplying clinical and analytical facilities to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, K.M., Hwang, WS., Jung, J.H. et al. Bioequivalence of Telmi plus V tablet 80/12.5 mg to Micardis plus tablet 80/12.5 mg (telmisartan/hydrochlorothiazide 80/12.5 mg). Journal of Pharmaceutical Investigation 45, 399–406 (2015). https://doi.org/10.1007/s40005-015-0185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-015-0185-0