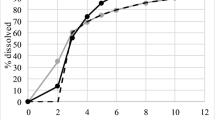
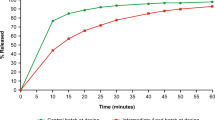
Valenta Pharm Co. compared the pharmacokinetic parameters in healthy volunteers of three prototype tizanidine dosage forms (delayed-release 6-mg tablets) and the reference drug sirdalud (2-mg tablets for a total dose of 6 mg, three tablets). This allowed the relative bioavailability of the three prototype tizanidine forms relative to sirdalud to be assessed. All three prototypes showed signs of delay based on MRT parameters of (2.217 ± 0.441) h for the sirdalud instant-release form and (6.529 ± 1.990), (5.951 ± 1.295), and (6.384 ± 2.339) h for prototypes T1, T2, and T3, respectively. High relative exposure levels of prototypes T1, T2, and T3 [AUC 103.80% (73.69 – 146.20), 124.14% (88.14 – 174.86), and 131.93% (93.66 – 185.82), respectively] with a significant decrease of C max (to 35.83, 38.78, and 40.79%, respectively) were demonstrated by analyzing the comparative bioavailability. A model pharmacokinetic study of the forms produced secondary modeling parameters that were similar to those obtained by an off-model method (89.19 – 122.71%). This confirmed that the developed model was acceptable for planning future clinical tests of these drugs.











Similar content being viewed by others
References
Instructions for Use of Sirdalud ® , Tablets, 2 mg and 4 mg [in Russian]; http: // www.grls.rosminzdrav.ru.
UK Public Assessment Report. Tizanidine 6mg Tablets; http: // www.mhra.gov.uk / home / groups / par / documents / websiteresources / con014794.pdf
V. G. Kukes, V. P. Fisenko, G. V. Ramenskaya, et al., Assessment of Drug Bioequivalency: Methodical Instructions [in Russian], Moscow (2008).
D. V. Reikhart, G. I. Baram, E. D. Gol?dberg, et al., Farmateka, No. 2 (98), 77 – 78 (2005).
D. V. Reikhart, Farmatsiya, 3, 5 – 8 (2010).
Acknowledgments
We thank Principal Investigator Dr. K. Anil, M. D., and Clinical Investigator Dr. S. Patil, MBBS, Human Pharmacology Unit, Clinigene International Ltd., Clinigene House, Tower I, Semicon Park.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 50, No. 6, pp. 26 – 35, June, 2016.
Rights and permissions
About this article
Cite this article
Kukes, V.G., Reikhart, D.V., Artnautov, V.S. et al. Comparative Bioavailability of Tizanidine in Three Dosage Forms (6-MG Delayed-Release Tablets, Sirdalud Preparation, and 2-MG Tablets). Pharm Chem J 50, 394–403 (2016). https://doi.org/10.1007/s11094-016-1458-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1458-2