Abstract
Purpose
To demonstrate the feasibility of continuous infusion of meropenem–vaborbactam to optimize the treatment of carbapenem-resistant Enterobacterales.
Methods
Report of a case of a Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae bloodstream infection comfirmed by whole genome sequencing and therapeutic drug monitoring (TDM) of meropenem.
Results
A patient with augmented renal clearance (ARC) went into septic shock caused by an ST11 KPC-3-producing K. pneumoniae bloodstream infection that was successfully treated with a continuous infusion of meropenem–vaborbactam at a dosage of 1 g/1 g q4h as a 4-h infusion. TDM confirmed sustained concentrations of meropenem ranging from 8 to 16 mg/L throughout the dosing interval.
Conclusion
Continuous infusion of meropenem–vaborbactam was feasible. It could be appropriate for optimizing the management of critically ill patients with ARC, as it resulted in antibiotic concentrations above the minimum inhibitory concentration for susceptible carbapenem-resistant Enterobacterales (up to 8 mg/L) throughout the dosing interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance is increasing globally, resulting in longer hospital stays, higher medical costs, and increased mortality [1]. Fortunately, novel β-lactam/β-lactamase inhibitor combinations have been designed to target carbapenemase-producing Enterobacterales, which are among the most threatening resistant bacteria [1, 2].
Meropenem–vaborbactam is one of these novel antimicrobials, combining a carbapenem and a cyclic boronic acid β-lactamase inhibitor (inhibiting Ambler class A and C β-lactamases) with potent activity against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacterales [2]. It was first approved by the US Food and Drug Administration (FDA) in 2017 with an optimized dosing regimen of 2 g/2 g (2 g of meropenem + 2 g of vaborbactam) q8h as a 3-h infusion to treat pathogens with MIC ≤ 8 mg/L [2, 3]. It is worth noting that dosage adjustment is recommended in patients with impaired renal clearance (< 50 mL/min/1.73 m2); however, there is no recommendation in patients with augmented renal clearance (ARC) [2,3,4]. For the latter, and in general for critically ill patients infected with carbapenem-resistant Enterobacterales, the use of continuous infusion (CI) could be interesting to limit therapeutic failures [5]. Indeed, like other β-lactams, meropenem–vaborbactam displays a predominantly time-dependent killing [2, 3]. Therefore, CI appears to be a promising dosing strategy to increase the likelihood of achieving the most optimized pharmacokinetic/pharmacodynamic (PK/PD) target [4, 8].
Here, we aimed to report the feasibility of meropenem–vaborbactam CI and to describe the meropenem pharmacokinetics in a patient with ARC and KPC-3-producing K. pneumoniae bloodstream infection (BSI) treated with this novel antimicrobial.
Materials and methods
Study design and setting
We reported the case of a patient hospitalized in Nimes University Hospital from 10/10/2022 to 9/12/2022, according to the CARE guidelines (for CAse REports).
Augmented renal clearance
We defined ARC as a creatinine clearance (calculated from a 24 h urine collection) greater than 130 mL/min/1.73 m2 with a normal serum creatinine.
Therapeutic drug monitoring
As meropenem and vaborbactam have shown comparable pharmacokinetic properties across varying degrees of renal dysfunction, therapeutic drug monitoring (TDM) of meropenem has been used as a surrogate for TDM of meropenem–vaborbactam [2]. Plasma concentrations of meropenem were determined using a high-performance liquid chromatography method [6].
Microbiology
Minimum inhibitory concentrations (MICs) were determined with the Sensititre™ EUMDRXXF plate (ThermoFisher Scientific™, Waltham, MA, USA), except for the MICs of ertapenem which were determined by Etest® (bioMérieux, Marcy-l’Etoile, France), and the MICs of cefiderocol which were determined with Liofilchem® ComASP® Cefiderocol (Liofilchem®, Roseto degli Abruzzi, TE, Italy) broth microdilution panel.
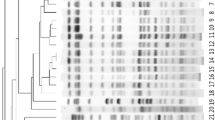
Carbapenemase was determined with the Xpert Carba-R test (Cepheid, USA, CA) in routine practice. Whole genome sequencing (WGS) was performed with the MiSeq System® using the Nextera® index kit (Illumina®, San Diego, CA, USA). Sequences were analysed by whole genome multilocus sequencing typing (wgMLST) with bioMérieux EPISEQ® CS (V1.2) software (19729 loci analysed, including 1515 in the core genome). K. pneumoniae strains were considered epidemiologically linked when their genomes contained less than 23 allelic differences [7].
Case presentation
In September 2022, a 46-year-old man, weighing 77 kg, with a medical history of Child C cirrhosis, was hospitalized for 3 weeks in Portugal, for variceal haemorrhage. In October 2022, he was admitted to a French tertiary hospital for ascites and jaundice. He was rapidly treated with ceftriaxone for 7 days for an undocumented spontaneous bacterial peritonitis. The rectal swab performed upon admission was positive for a KPC-producing K. pneumoniae (strain A, Table 1). Between day 7 and 27, hepatic encephalopathy and three episodes of acute variceal bleed occurred, requiring intensive care unit (ICU) admission, two endoscopic variceal ligations, a transjugular embolization of rectal varices, a transjugular intrahepatic portosystemic shunt, and an additional course of ceftriaxone for 10 days.
On day 27, his condition worsened due to septic shock, empirically treated with meropenem and amikacin. The blood cultures, drawn for fever 1 day prior, were positive for an extended spectrum β-lactamase (ESBL)-producing K. pneumoniae (strain B, Table 1). On day 29, the patient was still febrile and in septic shock, and the antimicrobials were switched to ceftazidime–avibactam (2 g/500 mg bolus followed by 2 g/500 mg q8h as an 8-h infusion). On day 33, the blood cultures drawn on day 32 were positive for KPC-producing K. pneumoniae (strain C, Table 1). On day 34, as the patient still had fever and sepsis, antimicrobials were switched to the one with the lowest MIC. Meropenem–vaborbactam 2 g/2 g q6h as a 3-h infusion (8 g/8 g/day due to ARC at 140 mL/min/1.73 m2) was started and catheters were replaced. No septic focus was evidenced on computerized tomography (CT) scan and positron emission tomography (PET) scan. Meropenem through concentrations on days 36, 39 and 40 were 6.5 mg/L, 3.8 mg/L and 2.7 mg/L, respectively. On day 40, the dosing regimen was optimized to 1 g/1 g q4h as a 4-h infusion corresponding to a CI of 6 g/6 g/day. Meropenem steady-state concentrations were 8.4 mg/L, 16.8 mg/L and 14.9 mg/L, on days 42, 47 and 54, respectively (Fig. 1). Blood cultures drawn on day 35, 36 and 47 were negative and the patient was discharged to home at the end of treatment (day 61). There was no evidence of adverse effects, such as hypersensitivity reaction, neurotoxicity or acute kidney injury during treatment.
Therapeutic drug monitoring of plasma meropenem concentrations in a critically ill patient with augmented renal clearance (> 130 mL/min/1.73 m2) treated with meropenem–vaborbactam at 2 g/2 g q6h as a 3-h infusion (extended intermittent infusion of 8 g/8 g/day), then at 1 g/1 g q4h as a 4-h infusion (CI of 6 g/6 g/day). Meropenem trough concentrations are represented by triangles and a dashed black line, and steady-state concentrations by triangles and a black line. Creatinine clearance is represented by squares and a black line, alanine transaminase (ALT) and aspartate transaminase (AST) are represented by circles and dotted black and grey lines, respectively, and C-reactive protein (CRP) is represented by squares and a dotted line
Interestingly, wgMLST showed that the KPC-3-producing K. pneumoniae strains A and C were not related (19.78% of sequence similarity) and belonged to international high-risk clones ST147 and ST11 (see Fig. 2 and Table 2). On the contrary, the KPC-3-producing K. pneumoniae strain C and the CTX-M-15-producing K. pneumoniae strain B isolated from the blood cultures were very similar (99.97%). They carried the same traT virulence gene on an IncFIB(K) plasmid. which encodes for an outer membrane protein of complement resistance and the same resistance genes on the IncFIA(HI1),IncR plasmid, except for the blaKPC-3 gene harboured by an IncN plasmid.
Discussion
To our knowledge, this is the first report of meropenem–vaborbactam administration by CI. The body of evidence suggests that CI of ß-lactams may be of interest in ICU patients with normal or ARC, to improve pharmacokinetic-target attainment, coverage of less-susceptible pathogens, bactericidal activity and outcomes [4, 8]. In accordance, we reported that only CI of meropenem–vaborbactam resulted in sustained concentrations above the MIC of susceptible pathogens throughout the whole dosing interval (100%ƒT > MIC), taking into consideration the 8 mg/L breakpoint [2, 3]. There is no optimized pharmacokinetic target available in the literature for meropenem–vaborbactam. However, it is likely that the optimized targets for other beta-lactams, i.e. a free drug concentration above fourfold the MIC throughout the whole dosing interval (100%ƒT > 4xMIC), can be applied [9]. We found that the CI of meropenem–vaborbactam achieved concentrations between 8 and 16 mg/L, thus reaching the optimized pharmacokinetic target of 100%ƒT > 4xMIC, for MIC up to 4 mg/L [10]. In addition, vaborbactam exhibits concentration–time profiles, suggesting that a pharmacokinetic target higher than 100%ƒT > 4xMIC could enhance bactericidal activity in infections due to KPC-producing Enterobacterales [2]. Therefore, even at the lowest MICs, CI of meropenem–vaborbactam could be of interest.
The CTX-M-15-producing K. pneumoniae (strain B) and the KPC-3-producing K. pneumoniae (strain C), involved in the BSIs, shared the IncFIB(K) plasmid with the traT gene that encodes an outer membrane protein of complement resistance, allowing the bacteria to escape the host immune system [11]. WGS suggested that strain C probably picked up the plasmid IncFIA(HI1), IncR with the blaKPC-3 gene, frequently described in Portugal [12, 13], from the KPC-3-producing K. pneumoniae (strain A) stool carriage. This case emphasizes the ability of K. pneumoniae to get new plasmids from other bacteria, which increases its resistance and virulence, and should prompt physicians to optimize drug selection and dosage in most severely ill patients [14].
Importantly, we reported that CI of meropenem–vaborbactam was feasible and safe when the daily dose was divided into six 4-h infusions in agreement with the manufacturer’s recommendations that meropenem–vaborbactam should not be infused for longer than 4 h, which is its stability limit at room temperature [2, 3, 15].
This study has limitations. First, the results presented in this case report need to be confirmed by larger studies. However, to our knowledge, this is the first clinical reference in the literature for the use of meropenem–vaborbactam CI. Second, we did not perform TDM of vaborbactam. Nevertheless, its pharmacokinetics seems to be comparable to that of meropenem [2]. Finally, we reported the effectiveness of meropenem–vaborbactam CI for bacteria with an MIC of 0.06 mg/L, and the effectiveness for bacteria with MICs up to 8 mg/L needs to be confirmed.
Conclusions
This case of successful microbiological and clinical treatment of a KPC-3-producing K. pneumoniae BSI in a critically ill patient with ARC, supports the use of meropenem–vaborbactam CI at a dosage of 1 g/1 g q4h as a 4-h infusion to achieve steady-state meropenem concentrations of 8–16 mg/L. Further investigations are warranted to confirm our results and to assess the effect of such a dosing regimen on the outcome of critically ill patients infected with carbapenem-resistant Enterobacterales.
Data availability
The authors consent to share the collected data with others. Data will made available by the authors, without undue reservation, immediately after the main publication and indefinitely.
References
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet Lond Engl. 2022;399:629–55.
Wenzler E, Scoble PJ. An appraisal of the pharmacokinetic and pharmacodynamic properties of meropenem–vaborbactam. Infect Dis Ther. 2020;9:769.
Summary of product characteristics: Vaborem [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/vaborem-epar-product-information_en.pdf. Accessed 23 Mar 2023
Silva CM, Baptista JP, Santos I, Martins P. Recommended antibiotic dosage regimens in critically ill patients with augmented renal clearance: a systematic review. Int J Antimicrob Agents. 2022;59:106569.
Rebold N, Lagnf AM, Alosaimy S, Holger DJ, Witucki P, Mannino A, et al. Risk factors for carbapenem-resistant enterobacterales clinical treatment failure. Goldberg JB, editor. Microbiol Spectr. 2023;11:e02647-22.
Legrand T, Vodovar D, Tournier N, Khoudour N, Hulin A. Simultaneous determination of eight β-lactam antibiotics, amoxicillin, cefazolin, cefepime, cefotaxime, ceftazidime, cloxacillin, oxacillin, and piperacillin, in human plasma by using ultra-high-performance liquid chromatography with ultraviolet detection. Antimicrob Agents Chemother. 2016;60:4734–42.
Jamin C, De Koster S, van Koeveringe S, De Coninck D, Mensaert K, De Bruyne K, et al. Harmonization of whole-genome sequencing for outbreak surveillance of enterobacteriaceae and enterococci. Microb Genom. 2021;7: 000567.
Roberts JA, Abdul-Aziz M-H, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent β-Lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194:681–91.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
The Infection Section of European Society of Intensive Care Medicine (ESICM), Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT), Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC), Abdul-Aziz MH, Alffenaar J-WC, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46:1127.
Ranjbar R, Kelishadrokhi AF, Chehelgerdi M. Molecular characterization, serotypes and phenotypic and genotypic evaluation of antibiotic resistance of the Klebsiella pneumoniae strains isolated from different types of hospital-acquired infections. Infect Drug Resist. 2019;12:603–11.
Rodrigues C, Bavlovič J, Machado E, Amorim J, Peixe L, Novais Â. KPC-3-producing Klebsiella pneumoniae in Portugal linked to previously circulating non-CG258 lineages and uncommon genetic platforms (Tn4401d-IncFIA and Tn4401d-IncN). Front Microbiol. 2016. https://doi.org/10.3389/fmicb.2016.01000.
Guerra AM, Lira A, Lameirão A, Selaru A, Abreu G, Lopes P, et al. Multiplicity of carbapenemase-producers three years after a KPC-3-producing K. pneumoniae ST147-K64 hospital outbreak. Antibiotics. 2020;9:806.
Hobson CA, Pierrat G, Tenaillon O, Bonacorsi S, Bercot B, Jaouen E, et al. Klebsiella pneumoniae carbapenemase variants resistant to ceftazidime-avibactam: an evolutionary overview. Antimicrob Agents Chemother. 2022;66:e00447-e522.
Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. Stability of meropenem and doripenem solutions for administration by continuous infusion. J Antimicrob Chemother. 2010;65:1073–5.
Acknowledgements
The authors acknowledge Stephanie Genieyz for technical support.
Funding
No funds, Grants, or other support was received.
Author information
Authors and Affiliations
Contributions
RL, TN, P-MB and CG were in charge of the patient. RL, PL-L, CM and AP contributed to the investigation and data collection. RL wrote the original draft. PL-L, TN, AP and AS critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RL has received consulting fees from MSD, payment or honoraria for lectures, presentations, speakers’ bureaus or educational events from BioMérieux, MSD, Pfizer and Shionogi, and support for attending meetings and/or travel from BioMérieux, MSD, Pfizer and Shionogi. AS has received consulting fees from Besins Healthcare and Karo Pharma, support for attending meetings and/or travel from Pfizer and MSD, and participates free of charge on advisory boards of Biofilm Control and CTX Laboratory. All other authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was not required by the local ethics committee for the case report. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
The patient provided written informed consent to participate in the study.
Consent to publish
The patient provided written informed consent for the publication of this report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Larcher, R., Laffont-Lozes, P., Naciri, T. et al. Continuous infusion of meropenem–vaborbactam for a KPC-3-producing Klebsiella pneumoniae bloodstream infection in a critically ill patient with augmented renal clearance. Infection 51, 1835–1840 (2023). https://doi.org/10.1007/s15010-023-02055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02055-2