Abstract
Purpose
Amongst all etiologic hospital-acquired infection factors, K. pneumoniae strains producing New Delhi metallo-β-lactamase (KP-NDM) belong to pathogens with the most effective antibiotic resistance mechanisms. Clinical guidelines recommend using ceftazidime/avibactam with aztreonam (CZA + AT) as the preferred option for NDM-producing Enterobacterales. However, the number of observations on such treatment regimen is limited. This retrospective study reports the clinical and microbiological outcomes of 23 patients with KP-NDM hospital-acquired infection treated with CZA + AT at a single center in Poland.
Methods
The isolates were derived from the urine, lungs, blood, peritoneal cavity, wounds, and peritonsillar abscess. In microbiological analysis, mass spectrometry for pathogen identification, polymerase chain reaction, or an immunochromatographic assay for detection of carbapenemase, as well as VITEK-2 system, broth microdilution, and microdilution in agar method for antimicrobial susceptibility tests were used, depending of the pathogens’ nature. CZA was administered intravenously (IV) at 2.5 g every eight hours in patients with normal kidney function, and aztreonam was administered at 2 g every eight hours IV. Such dosage was modified when renal function was reduced.
Results
KP-NDM was eradicated in all cases. Four patients (17.4%) died: three of them had a neoplastic disease, and one - a COVID-19 infection.
Conclusion
The combination of CZA + AT is a safe and effective therapy for infections caused by KP-NDM, both at the clinical and microbiological levels. The synergistic action of all compounds resulted in a good agreement between the clinical efficacy of CZA + AT and the results of in vitro susceptibility testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections, particularly those categorized as hospital-acquired, pose a global challenge, propelled by demographic factors such as a growing population over 70, compromised immunity linked to conditions like cancer and diabetes, and travels to regions with lower epidemiological standards. However, the primary factor is the escalating resistance of bacteria to antimicrobials, particularly among Gram-negative bacilli [1, 2]. This challenge is aggravated by the insufficient development of new antibiotics capable of addressing the evolving antibiotic resistance mechanisms that bacteria, particularly Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, are developing [3].
The breakthrough was the synthesis of carbapenems in the late 1970s, which to date are the primary weapon in treating infections caused by Enterobacterales possessing an extended-spectrum β-lactamases (ESBL) type resistance mechanism.
Klebsiella pneumoniae, one of the Enterobacterales family members, is commonly found in the oral cavity, nasal passages, skin, and gastrointestinal tract. The carriage rate of K. pneumoniae in the oral cavity varies from 5% in Western populations to 30% in Asia, and in the gastrointestinal tract, it ranges from 5 to 35% to even 60-70%, respectively. Although colonization is asymptomatic, carriage in the gastrointestinal tract may be a requisite for infection. K. pneumoniae is also a reservoir of antibiotic-resistance genes that can be transferred to other pathogens [4]. A significant proportion of such strains produce extended-spectrum β-lactamases. Those producing NDM belong to bacteria with the most effective antibiotic resistance mechanisms. The threat associated with the presence of KP-NDM is linked to its three characteristics: pathogen resistance to all or almost all available antibiotics, including colistin; ability to transfer resistance genes to other microorganisms (e.g., E. coli) through the localization of genes on plasmids and transposons; and high potential for spread within hospitals.
These characteristics limit the options for treating infections caused by KP-NDM. Colistin, gentamicin, tigecycline, and fosfomycin, used in the treatment of these infections, can lead to serious adverse effects, such as nephrotoxicity [5] and hepatotoxicity [6]. Karakonstantis et al. presented a therapeutic algorithm depending on the type of carbapenemase produced by carbapenem-resistant K. pneumoniae strains [7].
An unquestionable success of recent years has been the synthesis of new β-lactamase inhibitors like tazobactam, avibactam, vaborbactam, and relebactam, which, when combined with antibiotics like ceftalozane, ceftazidime, carbapenems have high efficacy against Enterobacterales capable of producing class A carbapenemases (K. pneumoniae carbapenemase [KPC], imipenem-hydrolyzing β-lactamase [IMI], Serratia marcescens enzyme [SME], Guiana extended-spectrum β-lactamase [GES], and class D (carbapenemase/oxacillinase [OXA]-48). However, class B carbapenemases so-called metallo-β-lactamases (MBLs: New Delhi metallo-β-lactamase [NDM], imipenemase metallo-β-lactamase [IMP], Verona integron-encoded metallo-β-lactamase [VIM] break down all β-lactamase inhibitors and combined antibiotics except aztreonam.
The guidelines of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [8] and the Infectious Diseases Society of America (IDSA) [9] recommend the use of ceftazidime/avibactam with aztreonam (CZA + AT) as the preferred treatment option for Enterobacterales producing NDM-type metallo-β-lactamases. However, the number of observations on such treatment regimen in infections caused by Gram-negative bacteria capable of producing MBL is limited. The most extensive paper on this topic included 82 patients with bacteremia caused by Enterobacterales NDM. It showed the therapeutic superiority of CZA + AT over other combination therapies [10].
Among the references provided by the authors above, only four comparable papers with notably fewer cases were identified. In the 2022 review article on utilizing new antibiotics encompassing CZA + AT therapy, only one report addressed the clinical follow-up of such treatment [11]. The conclusion of the extensive review by Karakonstakis et al. [7], summarizing therapeutic options for infections with multidrug-resistant strains of K. pneumoniae, P. aeruginosa, and A. baumannii, emphasizes the need for further research on synergistic antibiotic combinations and their administration regimens. According to the authors of this study, it aligns with the necessity of extending in vitro assessment of the treatment efficacy of the clinical one.
Therefore, this study aimed to report the clinical and microbiological outcomes of 23 patients diagnosed with KP-NDM infection treated with CZA + AT at a single center in Poland.
Materials and methods
Study protocol
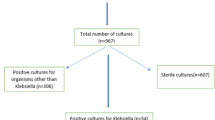
This paper is a retrospective study based on clinical material from the Military Medical Institute—National Research Institute in Warsaw, Poland, collected between June 2022 and November 2023. The study included 23 consecutive patients manifesting clinical symptoms of KP-NDM hospital-acquired infection.
Bacterial isolates identification and susceptibility testing
Bacterial isolates
The 23 KP-NDM isolates were derived from the following biological materials: urine (n = 8), lower respiratory tract (n = 7), blood (n = 3), peritoneal cavity (n = 2), wounds (n = 2), peritonsillar abscess (n = 1).
Bacterial identification
The strains were identified as K. pneumoniae by Matrix-Assisted Laser Desorption Ionization-Time Of Flight mass spectrometry (MALDI-TOF) (VITEK MS, bioMérieux, France).
Bacterial colonies were sampled from blood agar plates using a plastic loop with a capacity of 1 µl and transferred onto disposable target plates (VITEK MS, bioMérieux). Subsequently, one microliter of matrix solution (α-cyano-4-hydroxycinnamic acid, VITEK MS CHCA) was added. After air drying at room temperature, the target plates were analyzed using a mass spectrometer (VITEK MS). The main spectral profiles of the isolates were obtained under standard identification settings (positive linear mode, 2000–20,000 Da) using the VITEK MS IVD database version 3.2, comprising 15,556 microbial strains covering 1,316 species. Calibration and quality control for each set of 16 samples were conducted using E. coli ATCC 8739. The VITEK MS’s software calculated the confidence value and percentage probability to reflect the concordance of the observed spectrum with its database. Confidence values above 99% were interpreted as high-confidence results, between 60 and 99% as low-confidence results, and below 60% as unidentified. All strains were identified as K. pneumoniae with a confidence value of > 99%.
Detection of carbapenemases
The presence and nature of carbapenemase determinants were assessed by molecular testing of bacterial isolates using either a polymerase chain reaction (PCR) based platform GeneXpert System (Cepheid, USA) or a lateral flow immunochromatographic assay (RESIST-5 OOKNV, CORIS, BioConcept, Belgium).
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests were performed using the VITEK-2 automated system (bioMérieux, France), according to the manufacturer’s instructions, except for the colistin, fosfomycin, and cefiderocol determination. The minimal inhibitory concentration (MIC) of colistin was determined by micro-dilution in broth using the MICRONAUT MIC-Strip colistin (MMS) assay (Merlin Diagnostika GmbH, Germany). The MIC of fosfomycin was determined by microdilution method in Mueller Hinton Agar supplemented with 25 mg/L of glucose-6-phosphate using the AD Fosfomycin panel (Liofilchem®, Roseto degli Abruzzi, Italy), while the MIC of cefiderocol was determined by broth microdilution method using the ComASP® Cefiderocol panel, Liofilchem, Italy (Liofilchem®, Roseto degli Abruzzi, Italy).
The results were read according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [12].
Combination testing
The MIC test strip fixed-ratio method evaluated the synergy between CZA and AT (7–9). Respective MIC strips/scales were used to read MICs by placing them in each gradient’s position, and the FIC (Fractional inhibitory concentration) index was calculated. E. coli ATCC 25,922 was used as a quality control strain in all these experiments. The FIC was calculated and interpreted as described below:
-
FIC of agent A = MIC of agent A combination / MIC of agent A alone;
-
FIC of agent B = MIC of agent B in combination / MIC of agent B alone;
-
Cumulative FIC = FIC of agent A + FIC of agent B.
Synergy is interpreted when the FIC index is ≤ 0.5, addition corresponds to > 0.5 to ≤ 1, indifference corresponds to the FIC index > 1 to ≤ 4, and antagonism when the FIC index is > 4.0 [13, 14].
Antibiotic therapy
CZA was administered at 2.5 g every eight hours by intravenous (IV) infusion over 2 h in patients with normal kidney function. In patients with a creatinine clearance of 16–30 ml/min, CZA was administered at a dose of 0.75 g/0.1875 g every 12 h IV, and at lower clearance values, the dose was administered every 24 h. Aztreonam was administered at two grams every eight hours IV, while the antibiotic dose was halved in patients with a creatinine clearance of 10–20 ml/min.
Patients’ evaluation
The patients’ condition was continuously monitored with clinical examination, biochemical and microbiological tests. The clinical assessment involved monitoring the level of consciousness, body temperature, respiratory system function, circulatory system function, renal function, and - if the patient’s condition or reported symptoms required - other parameters. Blood samples were regularly collected for biochemical tests to monitor the dynamics of inflammatory markers and other parameters and objectively assess the patients’ condition. Microbiological diagnostics were also conducted.
Statistical analysis
The data were archived using Microsoft Excel. Statistical analysis was performed using R software version 4.3.1 (Vienna, Austria). Descriptive statistics were employed. Due to the relatively small sample size, the results are presented as the median and interquartile range.
Results
The general characteristics of the study patients
The general characteristics of analyzed patients are presented in Tables 1 and 2. Among the 23 investigated patients, a subgroup of eight individuals can be identified in whom the infection manifested as septic shock. These mentioned patients required aggressive pharmacotherapy, the administration of catecholamines, and mechanical ventilation. In the analyzed group, four patients (17.4%) succumbed to the illness. Among those who died, an additional complicating factor, independent of the infection, was a neoplastic disease (3 patients) and, in one case, COVID-19.
Blood laboratory tests
The C-reactive Protein (CRP), procalcitonin (PCT), and white blood cell count (WBC) were monitored to follow the dynamics of the infection. In all cases, a decrease in their levels from baseline values was observed during treatment. Creatinine and eGFR levels were determined to guide antibiotic dosage, whereas bilirubin levels were measured to reduce the antibiotics’ hepatotoxicity.
Microbiological investigations
Bacterial eradication from the site of infection occurred in all cases.
Table 3 contains data on those antibiotics to which antibiotic susceptibility of KP-NDM has been achieved to a greater or lesser extent. The bacterial strains from all the patients were 100% susceptible to cefiderocol. The microbiological results showed a 46% susceptibility to fosfomycin and trimethoprim/sulfamethoxazole, 31% to colistin and gentamicin, and 8% to amikacin and gentamicin.
MIC values of CZA + AT and results of the Etest MIC: MIC ratio method is displayed in Table 4. All the tested isolates were resistant to ceftazidime/avibactam and aztreonam separately. The combination (MIC test strip fixed-ratio method) of ceftazidime/avibactam and aztreonam displayed synergy against all the tested isolates with FIC of < 0.5, and none of the isolates had additive or antagonistic responses by this method (Table 4).
Discussion
In our study, all patients with symptoms of infection caused by KP-NDM treated with CZA + AT and pathogen eradication, defined as a negative result in microbiological testing, were achieved. This confirms the clinical effectiveness of such antibiotic therapy, even in patients with symptoms of septic shock. Combined treatment with CZA + AT for infections caused by Enterobacterales is recommended by ESCMID [8] and IDSA [9].
The benefits of such a combination stem from the fact that MBLs do not hydrolyze aztreonam, while CZA remains active against bacteria that can produce ESBLs and non-NDM carbapenemases.
Ceftazidime is a wide-spectrum cephalosporin, while avibactam is a β-lactamase inhibitor. Avibactam exhibits activity against all types of ESBLs, KPC, OXA48, and AmpC, but not MBLs (NDM, VIM, IMP). The ceftazidime/avibactam combination is effective against ESBLs, KPC, OXA-48, and AmpC, thereby restoring the efficacy of ceftazidime against Gram-negative bacteria. However, it does not work against MBL strains. Aztreonam is inactivated by ESBLs, KPC, and other cephalosporinases but remains stable against hydrolysis by MBLs. Thus, adding aztreonam to CZA enhances its activity by protecting aztreonam from the attack of ESBLs [15, 16].
In addition, the beneficial effect of such a combined set of antibiotics is the low MIC values of CZA + AZT shown in Table 3. Comparable findings were noted in a study encompassing 21 cases of K. pneumoniae NDM; specifically, in 8 samples, the MIC was below 0.13 µg/mL, while in the remaining cases, it measured 0.25 µg/mL [17]. However, this was only an in vitro study without assessing clinical outcomes. The same remark applies to the paper by Jayol et al. [15], who reported in vitro efficacy of the combination of ceftazidime-avibactam and aztreonam against isolates of carbapenemase-producing Klebsiella pneumoniae. This combination also proved effective in treating NDM-1-producing Klebsiella pneumoniae bacteremia in a neutropenic patient [18].
A comparison of the clinical efficacy of the combination of ceftazidime-avibactam and aztreonam versus treatment with colistin with fosfomycin and tigecycline or tigecycline with aminoglycosides or fosfomycin shows that the 30-day mortality rate for CZA + AT treatment was 19.2% and for other antibiotic combinations was much higher 44%. The most increased 30-day mortality was observed in patients treated with colistin-based regimens (59.3%) [10].
From our observations, the mortality rate was 17.4%. Four out of the analyzed patients died; three of them had a neoplastic disease, and one had a COVID-19 infection. This represents a lower mortality rate than in the Sree et al. [16] (29.9%) analysis but higher than in the study by Zhang et al. [19] (7.1%), although the latter analyzed hematologic patients.
The decrease in laboratory indicators of infection in our patients and the eradication of bacteria from the sites of infection show the effectiveness of the combination therapy.
Although the optimal pharmacokinetics/pharmacodynamics (PK/PD) values for the CZA + AT combination are not precisely defined, it can be assumed that these drugs have good tissue penetration in inflammatory conditions, especially when CZA + AT was given as a two-hour intravenous infusion, which ensures good concentration at the site of infection. Despite nearly half of the cases affecting the urinary tract, where antibiotics attain high concentrations, remarkable clinical efficacy was also observed in lung and abdominal infections, where the illness progressed to severe sepsis. This observation indirectly underscores the therapeutic effectiveness of the CZA + AT combination regimen.
No adverse clinically manifested side effects associated with the antibiotics used were observed.
Our study has some limitations. First of all, the number of observed patients is not large. It may limit our conclusions. Also, due to ethical considerations in line with current recommendations, the study lacks a comparative element with the most widely used antibiotic, colistin. In our analysis, the determination of MIC values for fosfomycin and cefiderocol was performed with a non-reference method. According to the EUCAST website communication [20], there is still no recommendation regarding an entirely reliable method for determining the susceptibility of microorganisms to cefiderocol. In our study, we employed, commercial tests that, according to the manufacturer, were validated by reference methods and held IVD certification.
Nevertheless, the unequivocal favorable results of the treatment entitle us to conclude that combined CZA + AT treatment is a safe, effective option for treating infections caused by K. pneumoniae having the ability to produce metallo-β-lactamases.
Conclusion
Our results support the conclusion that combined treatment of ceftazidime/avibactam and aztreonam is a safe and effective therapeutic option for infections caused by K. pneumoniae capable of producing metallo-β- lactamases, both at the clinical and microbiological levels. The synergistic action of all compounds resulted in an excellent agreement between the clinical efficacy of ceftazidime/avibactam and aztreonam and the results of in vitro susceptibility testing of K. pneumoniae.
Data availability
No datasets were generated or analysed during the current study.
References
Centers for Diseases Control and Prevention (2019) Antibiotics resistance threats in the United States, 2019. [ https://www.cdc.gov/drugresistance/biggest-threats.html. Accessed March 8, 2024
World Health Organization (2022) Global antimicrobial resistance and use surveillance system (GLASS) report 2022. [ https://www.who.int/publications/i/item/9789240062702. Accessed March 8, 2024
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Lindsay Grayson M, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18(3):318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Calderon-Gonzalez R, Lee A, Lopez-Campos G, Hancock SJ, Sa-Pessoa J, Dumigan A, McMullan R, Campbell EL, Bengoechea JA (2023) Modelling the gastrointestinal carriage of Klebsiella pneumoniae infections. mBio 14(1):e0312122. https://doi.org/10.1128/mbio.03121-22
Osorio J, Barreto J, Samboni CF, Cándelo LA, Alvarez LC, Benavidez S, Téllez RP, SantofimioD, Ramos JA, Gómez CA (2017) [Risk factors for acute kidney injury in patients treated with polymyxin B experience from 139 cases at a tertiary university hospital in Colombia]. Rev Chil Infectol 34(1):7–13. https://doi.org/10.4067/S0716-10182017000100001
Florescu DF, Qiu F, McCartan MA, Mindru C, Fey PD, Kalil AC (2012) What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis 54(5):670–680. https://doi.org/10.1093/cid/cir934
Karakonstantis S, Kritsotakis EI, Gikas A (2020) Treatment options for K. pneumoniae, P. Aeruginosa and A. Baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection 48(6):835–851. https://doi.org/10.1007/s15010-020-01520-6
Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo A, de Waele J, Daikos GL, Akova M, Harbarth S, Pulcini C, Gernacho-Montero J, Seme K, Tumbarello M, Lindermann PC, Gandra S, Yu Y, Bassetti M, Mouton JW, Tacconelli E, Rodriguez-Baño J (2022) European Society of Clinical Microbiology and Infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 28(4):521–547. https://doi.org/10.1016/j.cmi.2021.11.025
Tamma PD, Aitken SL, Bonomo RA, Mathers A, van Duin D, Clancy CJ (2022) Infectious Diseases Society of America 2022 Guidance on the treatment of extended-spectrum β-lactamase Producing enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat Resistance (DTR- P. aeruginosa). Clin Infect Dis 75(2):187–212. https://doi.org/10.1093/cid/ciac268
Falcone M, Daikos GL, Tiseo G, Bassoulis D, Giordano C, Galfo V, Leonildi A, Tagliaferri E, Barnini S, Sani S, Farcomeni A, Ghiadoni L, Menichetti F (2021) Efficacy of Ceftazidime-Avibactam Plus Aztreonam in patients with bloodstream infections caused by Metallo-β-lactamase–producing enterobacterales. Clin Infect Dis 72(11):1871–1878. https://doi.org/10.1093/cid/ciaa586
Windham S, Kollef MH (2022) How to use new antibiotics in the therapy of serious multidrug resistant gram-negative infections? Curr Opin Infect Dis 35(6):561–567. https://doi.org/10.1097/QCO.0000000000000858
The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical breakpoints - breakpoints and guidance (2003) Available from: https://www.eucast.org/clinical_breakpoints. Accessed March 8, 2024
Laishram S, Pragasam AK, Bakthavatchalam YD, Veeraraghavan B (2017) An update on Technical, interpretative and clinical relevance of Antimicrobial Synergy Testing methodologies. Indian J Med Microbiol 35(4):445–468. https://doi.org/10.4103/ijmm.IJMM_17_189
Doern CD (2014) When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52(12):4124–4128. https://doi.org/10.1128/JCM.01121-14
Jayol A, Nordmann P, Poirel L, Dubois V (2018) Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother 73(2):542–544. https://doi.org/10.1093/jac/dkx393
Sree RA, Gupta A, Gupta N, Veturi S, Reddy LS, Begum M, Shravani E, Challa HP, Reddy SS, Singamsetty A, Arumilli M, Reddy PN, Tirlangi PK (2003) Ceftazidime-Avibactam alone or in combination with Aztreonam versus Polymyxins in the management of carbapenem-resistant Klebsiella pneumoniae nosocomial infections (CAPRI study): a retrospective cohort study from South India. https://doi.org/10.1007/s15010-023-02094-9. Infection
Mikhail S, Singh NB, Kebriaei R, Rice SA, Stamper KC, Castanheira M, Rybak MJ (2019) Evaluation of the synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin, or Fosfomycin against Well-Characterized Multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 63(8):e00779–e00719. https://doi.org/10.1128/AAC.00779-19
Bocanegra-Ibarias P, Camacho-Ortiz A, Garza-González E, Flores-Treviño S, Kim H, Perez-Alba E (2020) Aztreonam plus Ceftazidime-Avibactam as treatment of NDM-1-producing Klebsiella pneumoniae bacteraemia in a neutropenic patient: last resort therapy? J Glob Antimicrob Resist 23:417–419. https://doi.org/10.1016/j.jgar.2020.10.019
Zhang L, Zhen S, Shen Y, Zhang T, Wang J, Li J, Lin Q, Xiao Z, Zheng Y, Jiang E, Han M, Wang J, Feng S (2023) Bloodstream infections due to Carbapenem-Resistant Enterobacteriaceae in hematological patients: assessment of risk factors for mortality and treatment options. Ann Clin Microbiol Antimicrob 22(1):41. https://doi.org/10.1186/s12941-023-00586-y
The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Cefiderocol susceptibility testing - an update (2023) Available from: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=554&cHash=46fef43d5bf84991a0af2d658deaf685. Accessed March 8, 2024
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: AG, ZR; acquisition of data: AG, KM; analysis of data: AG, DT, KM, WP; interpretation of data: AG, ZR, DT, WP, AC; draft of the work: AG, KM, DT; critical revision of the work: AG, ZR, DT, WP, AC. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee: Komisja Bioetyczna przy Wojskowej Izbie Lekarskiej w Warszawie (Approval No: KB 34/ 2023). The Ethics Committee has waived the requirement for the investigators to obtain a signed informed consent form from the participants due to the observational nature of this study and the fact that there is no harm related to the study participants.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guzek, A., Rybicki, Z., Tomaszewski, D. et al. Outcomes of 23 patients diagnosed with New Delhi metallo-beta-lactamase (NDM)-producing Klebsiella pneumoniae infection treated with ceftazidime/avibactam and aztreonam at a single center in Poland. Eur J Clin Microbiol Infect Dis (2024). https://doi.org/10.1007/s10096-024-04859-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10096-024-04859-y