Abstract
Purpose
Limited data are available on immunologic responses to primary pandemic H1N1 (2009) vaccination in recipients of allogeneic hematopoietic stem cell transplantation (HSCT) recipients. In 2009 serologic responses to either pandemic H1N1 (2009) vaccine (n = 36) or pandemic H1N1 (2009) infection (n = 2) were studied in 38 HSCT recipients.
Methods
Responses were measured with a standard hemagglutination-inhibition assay. Fourteen patients had active chronic graft-versus-host disease (cGvHD) at the time of vaccination/infection and seven patients had cGvHD in remission; 11 patients had no immunosuppressive therapy, and 27 patients were on immunosuppressive therapy. Nineteen patients (53%) responded to pandemic H1N1 (2009) vaccination. Two patients had pandemic H1N1 (2009) infection without prior vaccination, and one patient had severe pandemic H1N1 (2009) infection with acute respiratory distress syndrome despite prior single vaccination.
Results
Non-responders to pandemic H1N1 (2009) vaccination more often had cGvHD (65 vs. 53%) and received second- or third-line therapy (53 vs. 11%), while responders mostly had first-line therapy for cGvHD. While vaccine responders had no or single agent immunosuppressive therapy, non-responders frequently received moderate or intense immunosuppressive therapy. All vaccine recipients previously treated with rituximab were non-responders.
Conclusions
In summary, the overall response to pandemic H1N1 (2009) vaccination in HSCT recipients was modest. Patients receiving combined immunosuppressive therapy for steroid-refractory cGvHD barely responded to pandemic H1N1 (2009) vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients after allogeneic hematopoietic stem cell transplantation (alloHSCT) are at high risk for respiratory viral infections, such as influenza A, parainfluenza and respiratory syncytial virus (RSV). In spring 2009, a novel H1N1 influenza A virus caused a pandemic [1]. Following the pandemic, the seroprevalence of H1N1 antibodies was 36.9% in healthy people who had not received vaccination, with almost 70% of H1N1 (2009) infections being inapparent in healthy people [2]. Pandemic H1N1 (2009) influenza A infections may lead to severe pneumonia in children and young adults, with immunosuppression being an important risk factor [3–5]. Due to the profound immunodeficiency in patients after alloHSCT, pandemic H1N1 (2009) influenza A infection causes more severe respiratory disease in HSCT recipients than seasonal influenza A and B viruses, with graft-versus-host disease (GvHD) being an additional risk factor [6–10]. Therefore, prevention and prophylaxis of infections, including re-vaccination, are crucial in this patient group [11].
Limited data are available on the immunologic responses to primary pandemic H1N1 (2009) infection and pandemic H1N1 (2009) vaccination in patients with hematologic malignancies in general and in HSCT recipients in particular [12–15]. Here, we present the results of a retrospective analysis of pandemic H1N1 (2009) vaccination response in alloHSCT recipients and its correlation with clinical and immunological characteristics.
Patients and methods
Patients and clinical data
Thirty-eight alloHSCT recipients who received a pandemic H1N1 (2009) AS03-adjuvanted vaccine (n = 36; Pandemrix; GlaxoSmithKline, London, UK) or developed pandemic H1N1 (2009) infection (n = 2) during the fall of 2009 were included in this retrospective analysis. Blood samples for assessment of the vaccine response against H1N1 were obtained during regular visits at the outpatient clinic of the department of hematology and oncology at the University Hospital of Regensburg. The H1N1 vaccination was given either when the patient visited the outpatient department of the University Hospital or by a general practitioner. Twenty-two patients received only one dose of pandemic H1N1 vaccine, and 14 patients (39%) were vaccinated twice. Three patients had proven pandemic H1N1 (2009) influenza infection, one of whom had been vaccinated once previously against pandemic H1N1 (2009) virus. Medical records were reviewed, and relevant data at the time of vaccination were recorded, including gender, patient age, date of alloHSCT, date of pandemic H1N1 (2009) vaccination, time after transplantation, underlying disease, current remission status, donor type, HLA-match, stem cell source, and the conditioning regimen.
For all patients, the grade of prior acute and of prior and current chronic GvHD was documented. Acute GvHD (aGvHD) was graded according to the modified Keystone criteria, while chronic GvHD (cGvHD) was graded according to the National Institutes of Health standard [16, 17]. The intensity of immunosuppression required to control aGvHD was also noted (steroid-sensitive vs. refractory aGvHD). In addition, the type of onset of cGvHD (de novo, progressive, or quiescent onset) as well as the presence of thrombocytopenia at onset of cGvHD were documented. The number of treatment lines to control cGvHD and current intensity of immunosuppression at the time of vaccination were documented.
For the statistical analysis, three subcategories of immunosuppression were classified: (1) mild, indicating treatment with prednisone alone at a dose <0.5 mg/kg body weight (BW) per day; (2) moderate, indicating therapy consisting of prednisone alone at a dose ≥0.5 mg/kg BW per day and/or any other single agent of immunosuppressive therapy; (3) intense, consisting of two or more agents with or without prednisone in a dose ≥0.5 mg/kg BW per day according to Mitchell et al. [18]. Treatment with rituximab was documented separately, and it was noted whether rituximab was administered within the last 6 months prior to vaccination.
Methods
Lymphocyte subsets and immunoglobulins were measured in all but one patient at the time of vaccination. T cell subsets measured were the number of CD3+ cells, CD4+ T cells, CD4+CD45RA+ T cells, and CD4+CD45RA−CCR7+CD62L+ central effector memory T cells, as well as CD8+ T cells. B cells were determined by differentiating between naïve B cells (CD19+CD27−), immature B cells (CD19+CD21−CD27−), and memory B cells (CD19+CD27+). Analyses were performed by flow cytometry (FACS) of the peripheral blood, and the absolute values of these parameters were included in the statistical analysis. Moreover, the level of gamma-globulins in the serum was documented in relation to immunoglobulin-substitution if applicable.
Anti-H1N1 antibody titers were measured using the hemagglutination-inhibition (HI) assay before and at least once after vaccination (within 4–8 weeks after vaccination). No serum sample was obtained from three patients prior to vaccination. One patient showed no titer after vaccination and was classified as a non-responder. One patient was vaccinated twice: his titer after the first vaccination was 1:160 and after the second vaccination 1:1280. We therefore assumed a vaccination response because of the clear increase in titer after the second vaccination and due to the fact that the maximal baseline titer in all of the other patients prior to first vaccination was 1:40 and no other influenza vaccination was performed. The third patient showed a titer of 1:960, which was clearly positive and most likely not explained by a pre-existing titer. Therefore, this patient was regarded as a vaccination responder. A second sample was available for 23 of 38 patients, and a third sample was available for four of the 38 patients. Each serum sample was measured twice and the mean value included for analysis.
Antibody titers were measured by a HI assay as previously described [19]. Serum samples were pre-treated with receptor-destroying enzyme for inactivation of non-specific inhibitors. The sera samples were then titrated in twofold dilutions in phosphate-buffered saline at an initial dilution of 1:10 up to a final dilution of 1:1280. The strain A/California/7/2009 was used as the reference virus and adjusted to 4 HA units/25 μl, which was verified by back titration, and 25 μl of this virus suspension was added to each of the 96 wells. The plates were incubated at room temperature (RT) for 30 min. Freshly prepared 0.5% turkey red blood cells were added, followed by a further incubation at RT for 30 min. Human sera and an international H1N1 (2009) serum standard serving as positive controls and negative controls were included on each plate.
Titers were expressed as the reciprocal of the highest serum dilution at which hemagglutination was prevented. Samples that were negative according to the HI assay were assigned a titer of 1:5 for computational purposes in obtain fourfold increase of HI titers.
Seroconversion was defined as either a pre-vaccination titer of <1:10 together with a post-vaccination titer of ≥1:40, or a significant increase in HI titer by a factor of ≥4. Seroprotection was defined as a HI titer of 1:40 or higher.
For a more susceptible analysis of vaccination response, patients achieving any response to pandemic H1N1 (2009) vaccination were divided into very good responders, responders, and partial responders, respectively. Patients failing to achieve any response were classified as non-responders (titer <1:10). Partial responders achieved a titer (or an increase in titer) that was lower than 1:40. Very good responders were characterized with a titer (or an increase in titer) higher than 1:100, and responders showed a titer (or an increase in titer) between 1:40 and 1:100. The group of partial responders was included in the analysis since this subgroup would not have been captured by describing only seroconversion and protection rates.
Statistical analysis
The endpoint of the study was the response to pandemic H1N1 (2009) influenza vaccination in patients after alloHSCT. A second endpoint was the correlation of the vaccination response with clinical and immunological characteristics of these patients. Descriptive analyses were performed in Microsoft Excel (Microsoft, Redword, WA). Calculation of the impact of lymphocyte subsets and immunoglobulins were performed using SPSS ver. 15 (SPSS, Chicago, IL) by applying the Levene test and the t test. Differences in patient characteristics between responders and non-responders were evaluated by the chi-square test. In addition, a multi-regression analysis was performed to evaluate factors having an impact on the vaccination response, including the intensity of immunosuppression, severity of cGvHD, the application of rituximab, and B cell counts at the time of vaccination. Categorical variables were described as the number and relative frequency (%); continuous variables were summarized as averages and median.
Results
Patients
Thirty-eight HSCT recipients (28 unrelated and 10 related) were evaluated with regard to response to pandemic H1N1 (2009) vaccination. Thirty-six patients (95%) were vaccinated against H1N1 during the fall/winter of 2009, three patients had proven pandemic H1N1 (2009) infection, one patient developed severe pandemic H1N1 (2009) infection with acute respiratory distress syndrome (ARDS) on post-transplantation day 1902 despite prior single pandemic H1N1 (2009) vaccination 6 weeks prior to infection. The other two patients developed H1N1 infection on post-transplantation days 111 and 321, respectively. All patients with pandemic H1N1 (2009) infection received treatment with oseltamivir.
Fourteen patients (39%) received a second vaccination against pandemic H1N1 (2009) virus. The median time between vaccination and serum collection was 35 (range 14–70) days.
The grade of acute and chronic GvHD was documented for all patients, as depicted in Table 1. Twenty-seven patients (71%) had prior aGvHD, and none had active aGvHD at the time of pandemic H1N1 (2009) vaccination.
Twenty-one patients (55%) had cGvHD each time when tested. The cGvHD grade (NIH) at the time of H1N1 (2009) vaccination/infection was: mild in ten patients (26%), moderate in five patients (13%), and severe in one patient (3%). Fourteen patients (37%) had active cGvHD, seven patients (18%) had inactive cGvHD [defined as complete remission of cGVHD (n = 4) or absence of immunosuppression (n = 3)].
Table 1 shows the disposition of the different subcategories of immunosuppression among all patients receiving pandemic H1N1 (2009) vaccination.
Vaccine response
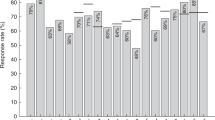
Seroconversion (pre-vaccination titer of <1:10 together with a post-vaccination titer of ≥1:40, or a significant increase in HI titer by a factor of ≥4) and seroprotection (HI titer of 1:40 or more) rate was 42% (n = 15/36) after the first pandemic H1N1 (2009) vaccination, increasing to a seroprotection rate of 47% (n = 17/36) after the second vaccination. Only 14 of 36 patients received a second vaccination. No severe adverse effect was attributable to pandemic H1N1 (2009) vaccination. Two patients had pandemic H1N1 (2009) infection without prior vaccination, and one patient developed severe pandemic H1N1 (2009) infection with ARDS despite a prior single pandemic H1N1 (2009) vaccination. This latter patient was considered to be a non-responder although a serum probe prior to infection was not available. Vaccination responders were further distinguished into very good responders (n = 15), responders (n = 2), and partial responders (n = 2) (Table 2; Fig. 1). Four partial responders were identified after the first vaccination. Three of these had a titer of 1:20, and one had a titer of 1:15, indicating failure to achieve seroconversion and/or seroprotection. Two patients in this group were vaccinated a second time: one showed an increase in titer from 1:15 to 1:40 (pr− > r), the second from 1:20 to 1:320 (pr− > vgr), indicating seroprotection.
Of the 19 patients responding to a first dose of pandemic H1N1 (2009) vaccine, seven (37%) received a second dose, and two additional patients showing only partial response to the first vaccination received a second vaccination with subsequent seroprotection. Of the 17 non-responding patients (29%), five showed no response to the second dose. Within the group of responders and very good responders, nine of 17 patients (53%) received a second H1N1 vaccine dose (Table 2).
Table 3 summarizes the clinical data for the groups of vaccination responders and non-responders.
Of the 19 responders to vaccination, nine were off immunosuppressive therapy (47%), five (26%) received mild immunosuppressive therapy, four (21%) received moderate immunosuppressive therapy, and only one patient (5%) received intense immunosuppressive therapy. In the group of non-responders (n = 17), the majority of patients were on mild or moderate immunosuppressive therapy: only two of 17 patients (12%) had had no immunosuppressant therapy, while seven (41%) and six (35%) patients received mild and moderate immunosuppressive treatment, respectively. Of the 17 patients not responding to pandemic H1N1 (2009) vaccination, two (12%) were receiving intense immunosuppressive therapy (Fig. 1). The group of very good responders and responders showed a similar disposition as the vaccination responders in general.
Categorizing the patients according to the underlying immunosuppressive therapy, we found that 82% of the patients not receiving immunosuppresssive therapy responded to pandemic H1N1 (2009) vaccination. In contrast, decreased responses were found among patients receiving immunosuppressive therapy, with the response rate being 42% among patients receiving mild immunosuppressive therapy, 40% among those receiving moderate immunosuppressive therapy, and 33% among those receiving intense immunosuppressive therapy.
The multi-regression analysis revealed that the intensity of the immunosuppression was the most important cofactor influencing vaccination response [exp (β) = 0.34, p = 0.064], but it failed to reach the significance level due to the limited number of patients. The response to pandemic H1N1 (2009) vaccination in correlation to intensity of immunosuppression is shown in Fig. 1.
Of the 17 non-responders, six (35%) had received prior rituximab therapy. Of the three patients with proven pandemic H1N1 (2009) infection, two were treated previously with rituximab, and one developed pandemic H1N1 (2009) infection despite vaccination 8 months after treatment with rituximab without further immunosuppressive treatment.
Interestingly, vaccination responders mostly had a history of grade 1 aGvHD (10/19, 53%) and rarely had grade 2 (2/19, 11%) and grade 3 aGvHD (1/19, 5%). In contrast, non-responders more frequently had a history of significant aGvHD (grade 1 in 4/17 cases, 24%; grade 2 in 6/17 cases, 35%; grade 3 in 2/17, 12%). Failure of the primary treatment of aGvHD and subsequent recurrence of aGvHD were associated with failure to respond to pandemic H1N1 (2009) vaccination (Table 3).
Non-responders more often had cGvHD at any time after transplantation (65 vs. 53%) and, applying the NIH grading of cGvHD, more frequently had moderate and severe cGvHD (responders: 89% with no or mild cGvHD and 11% with moderate cGvHD; non-responders: 76% with no or mild cGvHD; 24% with moderate or severe cGvHD). Active cGvHD at the time of pandemic H1N1 (2009) vaccination was more frequent in non-responders than responders (47 vs. 32%, respectively). While vaccination responders with cGvHD mostly had first-line therapy, non-responders received second- or third-line therapy for cGvHD (first-line therapy: 32 vs. 12%, respectively). Due to the limited number of patients, apart from the effect of time after transplantation, none of the factors reached the significance level (Table 3).
Responders had a significant higher number of naïve (CD19+CD27−) and memory B cells (CD19+CD27+) as well as gamma-globulins compared to non-responders (Table 4). The significant impact of naïve B cells and gamma-globulins persisted after patients receiving prior rituximab therapy had been excluded from the analysis.
Discussion
Our data demonstrate that alloHSCT recipients respond to vaccination against the pandemic H1N1 (2009) virus. We found a seroconversion and seroprotection rate of 42% (15/36), which is similar to rates reported on pandemic H1N1 (2009) vaccination in alloHSCT and autoHSCT patients by Issa et al. [13] and Engelhard et al. [15]. Previous studies of pandemic H1N1 (2009) vaccination in healthy people showed seroconversion in >90% within 21 days of vaccination [20, 21]. In contrast, Mackay et al. [22] found seroprotection in only 27% of patients with hematological malignancies compared to 50% in patients with solid tumors. Lu et al. [23] recently reported on patients receiving systemic immunosuppression and described a seroprotection rate of 76.2% in patients with systemic lupus erythematosus after inactivated monovalent A/H1N1 (2009) vaccination. The results of different vaccination trials indicate that the risk/benefit ratio favors vaccination and that the furthest time point from chemotherapy is the best [24, 25].
Garland et al. [12] described an impaired immune response to pandemic H1N1 (2009) infection in 13 patients with hematologic malignancies, including four alloHSCT recipients, and reported a response in six of 11 evaluable patients. In our cohort, one patient who had pandemic H1N1 (2009) infection after treatment with rituximab four months prior to infection failed to develop an antibody response following infection, indicating complete failure of serological response.
Fourteen of 36 patients (39%) received a second pandemic H1N1 (2009) vaccination. In this subcohort, two patients failing to respond with seroprotection after the first vaccination achieved seroprotection after the second dose, indicating a rather quantitative boost effect of the second vaccination. However, it can not be excluded that some of the patients failing to respond to the first vaccination, but who did not receive a second vaccination, would have achieved seroprotection in response to the latter. Similar results have been described by other groups [20]. In 2005, Ljungman et al. [26] found that compared to one dose of vaccine, two doses did not improve the vaccination response to influenza A/H1N1, A/H3N2, and influenza B in 70 patients with hematological malignancies. One dose of seasonal influenza A/H1N1 vaccine is highly immunogenic in adults, while two doses will probably be needed in children younger than 9 years [21]. Recent reports support the benefit of repeated H1N1 (2009) vaccination, indicating significantly increased HI titers in response to the first and second vaccination [15, 27].
The time post-transplantation was 367 days in responders (range 160–1406 days) and 540 days in non-responders (range 154–2893 days) due to a significant longer follow-up in patients with cGvHD in the non-responder group. Issa et al. [13] described an increasing vaccination response rate in correlation with the length of the time span after transplantation, reflecting improved immunoreconstitution; however, the latter is also impaired by cGvHD [28].
In our cohort, additional risk factors for failure of the vaccination response, in addition to the of cGVHD, were advanced treatment line of cGVHD, intensity of immunosuppression, severity of cGVHD, and a history of steroid-resistant acute GvHD. Although Issa et al. were not able to show a statistically significant influence of the presence of GvHD and concurrent immunosuppressive therapy (including prednisone) on the rate of seroprotection against pandemic H1N1 (2009) influenza virus, the trends were similar to our results: 59% of patients without GvHD had seroprotective titers, while 44% of those with aGvHD or cGvHD had seroprotective titers [13].
Of note, the pandemic H1N1 (2009) vaccination response rate in patients receiving immunosuppressive therapy for cGVHD was in the range of 42–33%, which is significantly lower than that in patients with systemic lupus erythematosus at a comparable intensity of immunosuppression, indicating an additional effect of the transplantation and cGvHD itself [23]. Issa et al. [13] found lower rates of seroprotective titers to pandemic H1N1 (2009) vaccination in patients receiving mycophenolate mofetil.
Rituximab is a human–mouse chimeric monoclonal antibody specific for CD20, a surface glycoprotein expressed on B lymphocytes. Prior rituximab therapy completely abrogated subsequent vaccination response in our cohort, which is in line with other results reported elsewhere [13, 22, 28, 29]. Moreover, a patient receiving rituximab as the sole immunosuppressive treatment 8 months before being vaccinated against pandemic H1N1 (2009) virus developed life-threatening pandemic H1N1 (2009) infection despite vaccination, indicating a long-lasting effect of rituximab. As observed in our cohort, the use of monoclonal antibodies, such as rituximab, has been associated with severe courses of influenza [6–8]. The effect of rituximab is in line with the association of low naïve and memory B cell counts with failure to respond to vaccination. B cells begin to recover at the very earliest 6 months after treatment, and they do not return to pretreatment levels for up to 1 year [30]. A very poor response to seasonal influenza vaccination in patients treated with monoclonal antibodies (rituximab or alemtuzumab) for lymphoma has been described by Ljungman et al. [26] and confirmed in patients with rheumatoid arthritis [31]. Whether recall antigens can generate an appreciable response to influenza vaccination in patients after rituximab therapy, as described by Takata et al., was not examined in our cohort [29].
The central role of B cells in the vaccination response has already been shown by Frasca et al., who demonstrated that intrinsic B cell defects in the elderly contribute to reduced antibody responses to influenza vaccine [32].
Interestingly, no correlation was detectable between the serological response rate and T cell counts of the peripheral blood. This is in line with a report by Greinix et al. [33] demonstrating a correlation of risk for infectious complications with peripheral B cell counts while T cell counts were not associated with the risk for infectious complications. The role of B cells is underlined by the impact of the immunoglobulin serum level on the vaccination response. The passive transfer of seroprotective HI titers by immunglobulin substitution can be excluded by the observation that patients receiving pandemic H1N1 vaccination following immunoglobulin substitution had no relevant HI titers prior to vaccination and that immunoglobulin substitution was a risk factor for failure to respond to vaccination.
In summary, the results of this retrospective analysis indicate an efficacy of pandemic H1N1 (2009) vaccination in patients after alloHSCT. Major risk factors for failure of the vaccination response are intensity of immunosuppression and cGvHD severity. Additional risk factors are a lack of naïve and memory B cells as well as low immunoglobulins at the time of vaccination. The complete failure to achieve a vaccination response after rituximab indicates the need for different strategies in the latter cohort.
References
Michaelis M, Doerr HW, Cinatl J Jr. An influenza A H1N1 virus revival—pandemic H1N1/09 virus. Infection. 2009;37:381–9.
Reinheimer C, Allwinn R, Doerr HW. Limited prevalence of influenza A/H1N1v antibodies: footprints of the pandemic of 2010. Infection. 2011;39:101–4.
Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15.
Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9.
Harris KM, Maurer J, Kellermann AL. Influenza vaccine—safe, effective, and mistrusted. N Engl J Med. 2010;363:2183–5.
Tramontana AR, George B, Hurt AC, et al. Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis. 2010;16:1068–75.
Wei JY, Chen FF, Jin J, et al. Novel influenza A (H1N1) in patients with hematologic disease. Leuk Lymphoma. 2010;51:2079–83.
Seiter K, Nadelman RB, Liu D, et al. Novel Influenza A (H1N1) in patients with hematologic malignancies. J Clin Oncol. 2010;28:27–9.
Espinosa-Aguilar L, Green JS, Forrest GN, et al. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: two centers experiences. Biol Blood Marrow Transpl. 2011;17:566–73.
Choi SM, Boudreault AA, Xie H (2011) Differences in clinical outcomes following 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood 117:5050–6.
Hilgendorf I, Jilg W, Einsele H, et al. Vaccination of allogeneic haematopoietic stem cell transplant recipients: report from the International Consensus Conference on Clinical Practice in chronic GVHD. Vaccine. 2011;29:2825–33.
Garland P, de Lavallade H, Sekine T, et al. Humoral and cellular immunity to primary H1N1 infection in patients with hematologic malignancies following stem cell transplantation. Biol Blood Marrow Transpl. 2011;17:632–9.
Issa NC, Marty FM, Gagne LS, et al. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transpl. 2011;17:434–8.
Redelman-Sidi G, Sepkowitz KA, Huang CK, et al. 2009 H1N1 influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J Infect. 2010;60:257–63.
Engelhard D, Zakay-Rones Z, Shapira MY, et al. The humoral immune response of hematopoietic stem cell transplantation recipients to AS03-adjuvanted A/California/7/2009 (H1N1) v-like virus vaccine during the 2009 pandemic. Vaccine. 2011;29:1777–82.
Filipovich AH. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:251–7.
Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Mitchell SA, Kline Leidy N, Mooney KH, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transpl. 2009;45:762–9.
Allwinn R, Geiler J, Berger A, et al. Determination of serum antibodies against swine-origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: how is prevalence rate of protecting antibodies in humans? Med Microbiol Immunol. 2010;199:117–21.
Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13.
Plennevaux E, Sheldon E, Blatter M, et al. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8.
Mackay HJ, McGee J. Villa D: evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J Clin Virol. 2011;50:212–6.
Lu CC, Wang YC, Lai JH, et al. A/H1N1 influenza vaccination in patients with systemic lupus erythematosus. Safety ImmunVaccine. 2011;29:444–50.
Pollyea DA, Brown JMY, Horning S. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28:2481–90.
Brydak LB, Calbecka M. Immunogenicity of influenza vaccine in patients with hemato-oncological disorders. Leuk Lymphoma. 1999;32:369–74.
Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. British J Haematol. 2005;130:96–8.
Gueller S, Allwinn R, Mousset S, et al. Enhanced immune response after a second dose of an AS03-adjuvanted H1N1 influenza A vaccine in patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011;17:1546–50.
Ljungman P, Cordonnier C, Einsele H, et al. Vaccination of hematopoietic cell transplant recipients. Guidelines. Bone Marrow Transpl. 2009;44:521–6.
Takata T, Suzumiya J, Ishikawa T, et al. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-Cell lymphoma after the administration of Rituximab-CHOP. J Clin Exp Hematopathol. 2009;49:9–13.
Pollyea DA, Brown JMY, Horning S. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28:2481–90.
van Assen S, Holvast A, Benne CA. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthr Rheum. 2010;62:75–81.
Frasca D, Diaz A, Romero M, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–84.
Greinix HT, Pohlreich D, Kouba M. Elevated numbers of immature/transitional CD21-B lymphocytes and deficiency of memory CD27 + B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2008;14:208–19.
Acknowledgments
The project was supported by a grant provided by the German “Sander Foundation” (DW, EH, ME) and a grant provided by the “BayImmunet” (ME). E. Holler and D. Wolff receive support by a grant provided by the Jose Carreras Foundation. The hemagglutination inhibition analysis (Robert Koch Institute) was performed without additional support. The authors thank Philipp Herzberg for support with the statistical analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roll, D., Ammer, J., Holler, B. et al. Vaccination against pandemic H1N1 (2009) in patients after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Infection 40, 153–161 (2012). https://doi.org/10.1007/s15010-011-0206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-011-0206-5