Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its resulting disease, coronavirus disease 2019 (COVID-19), has spread to millions of people worldwide. Preliminary data from organ transplant recipients have shown reduced seroconversion rates after the administration of different SARS-CoV-2 vaccination platforms. However, it is unknown whether different vaccination platforms provide different levels of protection against SARS-CoV-2. To answer this question, we prospectively studied 431 kidney and liver transplant recipients (kidney: n = 230; liver: n = 201) who received either the ChAdOx1 vaccine (n = 148) or the BNT-162b2 vaccine (n = 283) and underwent an assessment of immunoglobulin M/immunoglobulin G spike antibody levels. The primary objective of the study is to directly compare the efficacy of two different vaccine platforms in solid organ transplant recipients by measuring of immunoglobulin G (IgG) antibodies against the RBD of the spike protein (anti-RBD) two weeks after first and second doses. Our secondary endpoints were solicited specific local or systemic adverse events within 7 days after the receipt of each dose of the vaccine. There was no difference in the primary outcome between the two vaccine platforms in patients who received two vaccine doses. Unresponsiveness was mainly linked to diabetes. The rate of response after the first dose among younger older patients was significantly larger; however, after the second dose this difference did not persist (p = 0.079). Side effects were similar to those that were observed during the pivotal trials.
Similar content being viewed by others

Introduction
Since the emergence of the coronavirus disease 2019 (COVID-19) pandemic, several vaccine platforms have evolved and emergency use authorization has been filed for their use. Key platforms of these vaccines include mRNA and adenovirus vectors. Adenoviruses, retroviruses, and vaccinia viruses are typically used as carrier vehicles in viral vector vaccines [1].
Transplant recipients remain vulnerable to the development of severe COVID-19, with higher reported morbidity and mortality than the general population [2]. Solid organ transplant recipients and immunosuppressed individuals were excluded from phase 3 trials of all COVID-19 vaccines [3,4,5,6,7,8]. Studies have looked at the response of mRNA vaccines across solid organ transplant recipients, and showed diminished response. Which has led to recommending a third dose of the vaccine [9, 10]. Furthermore, the immune responsiveness across platforms may vary. No studies have explored the impact of different vaccine platforms on the generated immunity, especially in immunocompromised hosts. The primary objective of the study is to directly compare the efficacy and safety of two different vaccine platforms (i.e., BNT-162b2 vaccine versus ChAdOx1) in solid organ transplant recipients by measuring of immunoglobulin G (IgG) antibodies against the RBD of the spike protein (anti-RBD) two weeks after first and second doses. During this prospective study, we compared the immunogenicity of the two COVID-19 vaccine platforms prospectively.
Materials and methodS
Patient population and study design
Patients followed-up at the King Faisal Specialist Hospital and Research Centre who received two doses of either the BNT-162b2 vaccine or the ChAdOx1 vaccine were included in this study. Informed consent was obtained from all participants, and blood samples were obtained according to the follow-up schedule (Additional file 1: Appendix A).
The institutional ethics committee approved this study (RAC# 2211022). The key exclusion criterion for patients was known COVID-19 infection, multi-organ transplant and age < 18 years, receipt of the vaccine before transplant and history of rejection within 6 months preceding vaccine administration.
Antibody responses
The primary outcome was the measurement of immunoglobulin G (IgG) antibodies against the RBD of the spike protein (anti-RBD) two weeks after first and second doses (Additional file 1: Appendix A). The two-week time point was selected based on previous studies that indicated that antibody titers are expected to peak at those time points [11,12,13]. The anti-RBD was measured by semi-quantitative anti-spike serologic testing using the Roche Elecsys anti-SARS-CoV-2 spike enzyme immunoassay [14, 15]. Testing was performed according to the manufacturer’s instructions at a certified biochemistry testing hospital laboratory. The lower limit of detection of the assay was 0.4 U/mL; according to the test instructions, any level > 0.8 U/mL was considered positive. For the purposes of this study, we regarded any subject at or below 0.8 as negative. According to the manufacturer’s specifications, neutralizing antibodies were assessed via the SARS-CoV-2 surrogate virus neutralization test assay (GenScript). Horseradish peroxidase-conjugated spike RBD was incubated with serum and then moved to angiotensin-converting enzyme 2-coated wells. Interactions of RBD and angiotensin-converting enzyme 2 were blocked if neutralizing antibodies [16]were present in the serum. The surrogate virus neutralization test measures the total quantity of neutralizing antibodies in the sera [17]. A positive result was defined based on a neutralizing antibody limit of ≥ 30% neutralization/inhibition. At this limit, the negative and positive percent agreement with the conventional plaque reduction neutralization test 50 and plaque reduction neutralization test 90 assays was approximately 100%. The sensitivity and specificity of these assays were 93.80% and 99.4%, respectively, according to the manufacturer’s instructions. According to the kit specifications, individuals with neutralization less than 30% were considered negative for neutralizing antibodies.
Safety and adverse events
Our secondary endpoints were solicited specific local or systemic adverse events within 7 days after the receipt of each dose of the vaccine, and unsolicited adverse events within 30 days after the receipt of the second dose of the vaccine (Additional file 11: Appendix A).
The study team members contacted all participants within 1 week of the receipt of each dose by phone to collect any adverse events. The data were collected at each scheduled visit (Additional file 1: Appendix A) to assess episodes of acute allograft rejection, hospitalization, other adverse events, or COVID-19 infection during the entire duration of the study.
Statistical analysis
The immunogenicity analysis was performed two weeks after the receipt of the first dose and 2 weeks after the receipt of the second dose for patients who received both vaccine doses and returned for follow-up. A safety analysis was performed for all patients, regardless of the number of doses administered. Demographic and safety analyses were performed using descriptive statistics. The primary outcome was vaccine immunogenicity assessed according to the anti-RBD titer two weeks after each dose of the vaccine, and will be further adjusted using propensity score analysis. A positive anti-RBD response was defined as > 0.8 U/mL. Univariate analyses were performed to determine factors impacting the development of a positive anti-RBD titer using the χ2 or Fisher’s exact test for categorical variables and we analyzed for changes in the lab parameters between screening and before the 2nd dose, and between screening and after the 2nd dose via t-tests. Statistical significance was defined as p < 0.05. All statistical analyses were performed using Stata version 17.0 (College Station, TX, USA).
The primary immunogenicity endpoint was considered the most important factor determining the necessary number of participants for this study. Furthermore, the endpoint was assumed to be binary for sample size calculations; that is, the recruited participant either did or did not achieve a sufficient antibody titer level 2 weeks after the second dose.
Multivariable logistic regression analyses were performed to simultaneously investigate the relationship between subgroups and the rate of immunogenicity. Similar analyses were performed to determine the efficacy outcomes (i.e., infection).
Results
Patient characteristics
Our cohort included 431 participants. Of these, 283 received the BNT-162b2 vaccine and 148 patients received the ChAdOx1 vaccine (230 kidney transplant recipients and 201 liver transplant recipients). The median age was 51.3 (± 16.2) years and 295 (68.4) were male. None reported a known history of COVID-19 prior to vaccination. All patient had stable graft function at the time of the vaccine. The baseline characteristics of the patients are described in Table 1. No significant differences in baseline characteristics were noted except for age (p > 0.00001) (Table 1).
Immunosuppression
The primary immunosuppressive regimen in the majority of the cohort composed of tacrolimus, mycophenolate and prednisone 235 (54.5%). With 408 (94.6%) of the patients were on tacrolimus as the cornerstone immunosuppressant. The immunosuppression intensity had the same impact on the vaccine response rate according to the neutralizing antibody (Table 1).
Vaccine immunogenicity according to the neutralizing antibody
All patients were screened for COVID-19 before enrollment. Baseline laboratory test results and graft function were also assessed. There was no difference between patient’s laboratory parameters from baseline and two weeks following each dose of the vaccine (Table 2).
Factors associated with a lack of response to the vaccine
Factors previously reported to have affected seroresponse such as younger age, gender and time from transplantation were not clearly associated with response in our cohort. However, diabetes and triple immunosuppressive therapy appears to have significantly affected the response (Table 3).
A multivariable logistic regression was used including the same factors and demonstrated a pseudo R-square value of 0.23. Triple immunosuppressive therapy and age were identified as significant contributors for lack of response to the vaccine after the second dose with those receiving triple therapy having 92% reduced odds of a response and the per unit (year) increase in age associated with a 5% reduction in the odds of a response (Table 4).
Anti-RBD levels by vaccine type
In our cohort, the response rate after the first vaccine dose appeared to be higher with Pfizer vaccine (P < 0.0001). However, this elevation did not persist until after the second dose (P = 0.863) (Table 5).
However, type of organ transplant significantly affected the response rate in our cohort (p = 0.002) (Table 6).
Change in spike antibody serology
The median antibody level before the second dose was 0.4 and after the second dose was 82.2. The median change in antibodies from before the second dose to after the second dose was 10.1
Incidence of COVID-19
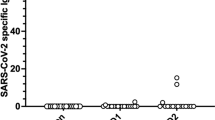
A total of 45 cases of COVID-19 were confirmed by polymerase chain reaction in this cohort; these cases occurred in 19 of 148 participants who received the AstraZeneca vaccine and in 26 of the 283 participants who received the Pfizer-BioNTech vaccine. P = 0.213 (Fig. 1; Table 7).
Vaccine safety and other outcomes
No evidence of graft dysfunction or rejection, or any other form of abnormality was observed in the entire cohort as evident by routine laboratory monitoring (Table 2). There were no significant changes in liver enzymes or liver function test results in the liver transplant population throughout the study period. There were no changes in serum creatinine levels in the kidney transplant population that necessitated any kidney allograft biopsy or further investigation. All side effects that occurred were grade 1 (mild) [18, 19], no medical intervention/therapy required. in this study were consistent with what’s been reported previously. Pain at injection site and fatigue occurred mainly with ChAdOx1 vaccine (Table 8).
Discussion
A key strength of our study is the head-to-head evaluation and comparison of the serologic response to the BNT162b2 mRNA and ChAdOx1 vaccines against COVID-19 in a large transplant cohort in a prospective fashion. Our key finding is that both vaccine platforms provide comparable anti-Spike levels against COVID19 infection, even after adjusting with propensity score matching. On the other hand, previously reported factors that may have an impact on vaccine responsiveness were not evident in our cohort [20, 21].
It is not yet clear whether these antibody responses will be adequate to protect transplant recipients from symptomatic COVID-19. Associations between neutralizing activity and clinical protection were not evaluable in this study due to the small number of breakthrough infection in the cohort.
Another point of originality of our study is that, we showed that both vaccine platforms were safe, and have comparable side effect profile. We have also noticed that BNT-162b2 vaccine may produce higher titers numerically, especially after first dose, this effect did not persist after the second dose. A previous study examined the outcomes of the Ad26.COV2. S vaccine compared to those of the mRNA vaccine; only 2 of 12 participants who received a single dose of the Ad26.COV2. S vaccine had a detectable anti-RBD antibody response, which was significantly fewer than the observed number of recipients with a detectable anti-RBD antibody response who received the mRNA vaccine series. Additionally, the titers achieved by the Ad26.COV2. S groups were significantly lower than those achieved by the mRNA group [22]. One potential explanation of the lower titer level after the first dose in the ChAdOx1 arm is that, in clinical trials, antibody titers usually peak at 21 days after receipt of the first dose [23], our study protocol measures the titers two week after each dose of the vaccine.
During SARS-CoV-2 mRNA and virus vector vaccine studies involving the general population, seroconversion was observed in almost all patients [3, 4, 6,7,8, 15, 24]. However, as expected, the response rate was lower in our cohort than it was in the general population; this finding is consistent with the available data in the field [25,26,27,28]. Considering only the humoral response, spike-specific antibodies developed in only 29.9% of patients in our population, which is a bit lower than general population, and those with other immunocompromising conditions [29]. However, studies have reported a 37.5% antibody response rate after the second dose of the BNT162b2 vaccine. Boyarsky et al. reported a higher seroconversion rate of 54% for patients who received either the mRNA-1273 vaccine (Moderna®) or the BNT162b2 vaccine (Pfizer), both of which are mRNA vaccines [30]. Although no consensus on what threshold should be considered as protective immunity. In general, antibody levels were well below what has been reported in immunocompetent subjects.
It has been reported that the immune response to the vaccines was also impacted by the immunosuppressive protocol used [31, 32]. Some studies have addressed that anti-metabolite use (mycophenolate and azathioprine) are linked to poorer humoral responses to COVID-19 vaccines after SOT [33, 34]. Yet, the impact was consistent across vaccination platforms in our cohort. Moreover, we found that the odds of seropositivity among SOT patients receiving triple immunosuppressive regimen was lower compared to those receiving only 1 drug, irrespective of the pharmacological class. This implicates that the net state of immunosuppression, is the main predictor of poor humoral responses after SOT rather than a particular medication. We also found that seropositivity in kidney transplant recipients was lower than that of liver transplant recipients, which could also be explained by the intensity of immunosuppressive regimen used across organs.
It has also been observed that, in SOT recipients, the odds of seropositivity in patients who were vaccinated within 1 year after transplantation was lower than those who received the vaccines after the 1st year of transplantation [21]. This effect was not evident in our population, and was consistent across vaccine platforms.
The safety of both vaccine platforms especially vector vaccines in solid organ transplant recipients was another point of concern amongst healthcare providers. Our findings match those reported in the original trials of the BNT162b2 vaccines. Pain at the injection site, fatigue, and headache were the most common symptoms experienced by healthy adults and those with stable, chronic medical conditions [31, 32]. None of the subjects in our large cohort experienced serious adverse events such as thrombocytopenia nor severe hypersensitivity reaction similar to what have been published [32, 35,36,37,38] Those findings shall eliminate hesitancy or preference of a particular vaccine platform over the other.
However, the concern remains whether the antibody titers correlate with the clinically meaningful protection. Therefore, the clinicians should inform the patients that the immune response following vaccination may not provide a full protection against COVID19 infection.
To the best of our knowledge, this is the first study that directly compared the efficacy of different vaccine platforms in solid organ transplant recipients. Our results suggest that solid organ transplant recipients should not be limited to COVID-19 vaccinations with mRNA platforms despite of the observed of the suppressed efficacy of viral vector vaccines, and that their antibody titers should be routinely checked to assess the response. At this point, the focus should continue to be vaccinating the family members and caregivers of solid organ transplant recipients as part of a cocooning strategy, which is a well-known method of protection when the target population cannot be vaccinated or is at risk for having a low response rate.
Limitations of this study include, lack of an immunocompetent control group, and lack of exploration of memory B-cell or T-cell responses. We also did not evaluate neutralizing antibody titers against the Delta or Omicron SARS-CoV-2 variants. Given that those variants were not reported at the time of the conduct of the study. Moreover, vaccine efficacy against these two variants is likely reduced [39,40,41,42,43,44].
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ANC:
-
Absolute neutrophil count
- AE :
-
Adverse event
- ALT:
-
Alanine aminotransferase
- ANOVA :
-
Analysis of variance
- AST:
-
Aspartate aminotransferase
- AZA:
-
Azathioprine
- β − ηΧΓ:
-
Beta-human chorionic gonadotropin
- BCAR:
-
Biopsy-proven acute rejection
- BP :
-
Blood pressure
- BMI:
-
Body mass index
- CNI :
-
Calcineurin inhibitor
- CRF:
-
Case report form
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- CIOMS:
-
Council for International Organizations of Medical Sciences
- CSA:
-
Cyclosporine
- CMV :
-
Cytomegalovirus
- DNA:
-
Deoxyribonucleic acid
- ECG:
-
Electrocardiogram
- eCRF:
-
Electronic case report form
- EBV:
-
Epstein-Barr virus
- eGFR:
-
Estimated glomerular filtration rate
- EMA:
-
European Medicines Agency
- FSH:
-
Follicle-stimulating hormone
- GCP:
-
Good clinical practice
- HBc Ab :
-
Hepatitis B core antibody
- HBV:
-
Hepatitis B virus
- HIV :
-
Human immunodeficiency virus
- HIV :
-
Human immunodeficiency virus
- ICU:
-
Intensive care unit
References
Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963.
Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–303.
Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021;384(19):1824–35.
Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78.
Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8(8):e474–85.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16.
Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2021;384(16):1576–7.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transpl. 2021.
Karaba AH, Zhu X, Liang T, et al. A Third Dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. medRxiv. 2021.
Almendro-Vazquez P, Laguna-Goya R, Ruiz-Ruigomez M, et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021;17(12): e1010211.
Al-Sadeq DW, Shurrab FM, Ismail A, et al. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naive and previously infected individuals. J Travel Med. 2021;28(8).
Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to COVID-19. N Engl J Med. 2021;385(26):2413–20.
Patel EU, Bloch EM, Clarke W, et al. comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2).
Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. medRxiv. 2020.
Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–8.
Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5).
Helling M, Venulet J. Drug recording and classification by the WHO research centre for international monitoring of adverse reactions to drugs. Methods Inf Med. 1974;13(3):169–78.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.
Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121(8):3109–19.
Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis. 2022.
Boyarsky BJ, Chiang TP, Ou MT, et al. Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. 2021;105(8):e82–3.
Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–9.
Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–54.
Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–39.
Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021.
Benotmane I, Gautier-Vargas G, Gallais F, et al. Strong antibody response after a first dose of a SARS-CoV-2 mRNA-based vaccine in kidney transplant recipients with a previous history of COVID-19. Am J Transpl. 2021.
Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–9.
Benotmane I, Gautier-Vargas G, Gallais F, et al. Strong antibody response after a first dose of a SARS-CoV-2 mRNA-based vaccine in kidney transplant recipients with a previous history of COVID-19. Am J Transpl. 2021;21(11):3808–10.
Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–6.
Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–2.
Grupper A, Katchman H. SARS-CoV-2 vaccines: safety and immunogenicity in solid organ transplant recipients and strategies for improving vaccine responses. Curr Transpl Rep. 2022;9:35–47.
Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transpl. 2021;21(8):2719–26.
Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–8.
Strobel D, Haberkamp S, Zundler S. Portal vein thrombosis due to vaccine-induced immune thrombotic thrombocytopenia (VITT) after COVID vaccination with ChAdOx1 nCoV-19. Ultraschall Med. 2021;42(5):551–2.
Choi PY. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385(3): e11.
Lai KY, Au SY, Fong KM. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385(3): e11.
Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101.
Ma C, Sun W, Tang T, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: Evidence from an outbreak in Yunnan, China, 2021. Vaccine. 2022.
Pormohammad A, Zarei M, Ghorbani S, et al. Effectiveness of COVID-19 vaccines against delta (B.1.617.2) variant: a systematic review and meta-analysis of clinical studies. Vaccines (Basel). 2021;10(1).
Emani VR, Reddy R, Goswami S. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(25):e92.
Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines (Basel). 2022;10(3).
Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376: e069761.
Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–45.
Acknowledgements
Not applicable.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the Transplant International.
Funding
This is an investigator initiated study. No funding has been received in relation to any part of the study.
Author information
Authors and Affiliations
Contributions
AAA, conception & design of the work; & the acquisition, analysis, & interpretation of data; & the creation of new software used in the work; & have drafted the work & substantively revised it. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. TA, conception & design of the work; & substantively revised it. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. HA, conception & design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. KA, conception & design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. EDV the acquisition, analysis, & interpretation of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. MAA Contributed to drafted the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. LF, the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. AA the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. DH the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. SA, Contributed to drafted the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. SA, Design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. AU, conception & design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. AA, conception & design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. KB, conception & design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. RA, Design of the work. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. KAHM, the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. BE, the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. EA, the acquisition of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. HA, the acquisition, analysis, & interpretation of data; approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. MA-A, Manuscript review. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. Prof. DCB, Manuscript review. approved the submitted version (and any substantially modified version that involves the author's contribution to the study); agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been reviewed and approved by institutional review board of Office of research affairs (ORA) and Research Ethics Committee (REC) of King Faisal Specialist Hospital and Research Centre with RAC # 2211022. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all subjects/participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Appendix A:
Schedule of Assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ajlan, A.A., Ali, T., Aleid, H. et al. Comparison of the safety and immunogenicity of the BNT-162b2 vaccine and the ChAdOx1 vaccine for solid organ transplant recipients: a prospective study. BMC Infect Dis 22, 786 (2022). https://doi.org/10.1186/s12879-022-07764-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07764-x