Abstract
Introduction
Hemophagocytic syndrome represents a severe hyperinflammatory condition by activated macrophages. Leading viral triggering agents are Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus.
Materials and methods
We present a patient with Wegener’s granulomatosis on azathioprine and prednisone medication, who developed a life-threatening hemophagocytic syndrome. Positive plasma polymerase chain reaction (PCR) with negative serology revealed a primary, disseminated infection with herpes simplex virus-1 as the triggering pathogen. After treatment with acyclovir, high-dose steroids, immunoglobulins, and etoposide, the patient recovered.
Conclusion
Early diagnosis of potentially underlying infections of hemophagocytic syndrome influences the therapeutic approach. It is important to consider a variety of infectious agents, particularly in immunosuppressed individuals. The reported case emphasizes the importance of screening for herpes simplex virus 1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophagocytic syndrome represents a severe hyperinflammatory condition characterized by prolonged fever, cytopenia, hepatosplenomegaly, and hemophagocytosis by activated macrophages. The acquired hemophagocytic syndrome was first described in 1979 by Risdall et al. [1] in association with viral infections following organ transplantation. Subsequently, it became clear that, also, non-viral pathogens, including bacteria, protozoae, and fungi, can trigger hemophagocytic syndrome [2]. Leading viral triggering agents are Epstein–Barr virus (EBV), cytomegalovirus (CMV), and adenovirus. To the best of our knowledge, we present here the first case of a hemophagocytic syndrome in an adult caused by a primary infection with herpes simplex virus type 1 (HSV-1).
Case
The 57-year-old patient was diagnosed with c-ANCA-associated Wegener’s granulomatosis in 1999. Progressive glomerulonephritis resulted in renal failure requiring dialysis 8 years later. In July 2008, the patient developed progressive malaise with elevated inflammation markers (Table 1) and recurrent fevers. Since a relapse of Wegener’s granulomatosis was suspected, prednisone was augmented from 10 to 50 mg/daily, while azathioprine was maintained at 150 mg/daily. Despite the increased immunosuppressive therapy, the patient’s clinical condition deteriorated rapidly, making a relapse of the Wegener’s granulomatosis unlikely. The patient developed right-sided abdominal pain and the computed tomography (CT) scan was suspicious for cholecystitis. Since empiric antibiotic treatment was not successful, cholecystectomy was performed 2 weeks later, showing chronic cholecystitis.
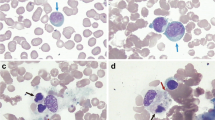
After surgery, fever persisted and the patient developed severe pancytopenia. Consequently, immunosuppression with azathioprine was stopped and the patient was referred to our hospital for further investigations. The patient required mechanical ventilation because of reduced consciousness. Laboratory examinations on admission showed severe pancytopenia (Table 1) and highly elevated values for ferritin, soluble IL-2 receptor, and neopterin, raising the suspicion for hemophagocytic syndrome. Bone marrow examinations showed marked hypocellularity and an increase of macrophages with hemophagocytosis (Fig. 1).
Microbiological investigations showed no evidence for infection with CMV, EBV, human parvovirus B19, hepatitis A, B, C, and HIV. Blood and stool cultures remained negative. A highly positive DNA polymerase chain reaction (PCR) for HSV-1 was found in the peripheral blood with 157 million genome copies/mL. The only clinical sign for a HSV-1 infection was a vesicular eruption on the buccal mucosa, where HSV-1 DNA could be detected as well. The PCR was done according to the primer sequences published by Pevenstein et al. [3] using a Taqman® MGB probe with the Applied Biosystems 7300 real-time PCR system. IgM and IgG serology for HSV was negative on admission, being compatible with a primary HSV-1 infection. Treatment with acyclovir was initiated. HSV was also found in the cerebrospinal fluid (CSF, 6,000 genome copies/mL). However, CSF revealed normal cell count (1 cell/μL), protein, and glucose levels. Neither magnetic resonance imaging (MRI) nor electroencephalography showed typical signs of HSV-encephalitis. The detection of HSV-1 genome in the CSF being 5log lower than in the plasma was interpreted as a spillover from plasma rather than as intracerebral HSV-1 replication.
Treatment for hemophagocytic syndrome was initiated with 2,500 mg methylprednisolone daily for 3 days, followed by three further doses of 100 mg prednisone daily. Due to insufficient response to the steroids, etoposide (100 mg loading dose, consecutively reduced to 20 mg/day) and intravenous immunoglobulins (40 g for 3 days) were added to the therapy.
Although the activity markers of the hemophagocytic syndrome declined rapidly during the first days of treatment, they did not reach normal levels. Likewise, severe pancytopenia persisted, requiring regular substitution of erythrocytes and platelets. After 4 weeks, the patient’s condition slowly improved, and he was weaned off the ventilator. Acyclovir was given for 8 weeks and resulted in a complete suppression of HSV in plasma. Nine weeks after admission, the patient still presented signs of severe encephalopathy and bone marrow biopsy showed ongoing, although less intense, signs of hemophagocytosis. Thus, another course of high-dose steroids and intravenous immunoglobulins was administered. Thereafter, the patient’s neurological status improved considerably and in the subsequent bone marrow microscopy, a distinct improvement of the hemophagocytic activity was documented. Repeated HSV serology revealed a seroconversion. The patient was discharged from hospital after 13 weeks and he remained in good clinical condition since. One year later, he underwent successful kidney transplantation.
Discussion
To the best of our knowledge, we report here the first case in which hemophagocytic syndrome was caused by a primary HSV-1 infection unambiguously documented by a seroconversion in the course of the disease. So far, only six adults with hemophagocytic syndromes associated with HSV infections are described in the literature. One case was associated with HSV-1 as diagnosed by increasing antibody titer, suggesting HSV-1 reactivation [4]. Three cases had HSV-2 [5–7] and in two cases, the virus subtype was not specified [1, 8]. Several factors may account for the rarity of a primary HSV-1 infection as a cause of hemophagocytic syndrome. First, it is unusual that a 57-year-old patient has a negative HSV-1 serology. The prevalence of HSV-1 infection increases gradually with age; by the fifth decade of life, positive HSV-1 antibodies can be found in up to 90% of the population [9]. Thus, during adulthood, the reactivation of HSV-1 is more frequent than primary infection.
Second, primary HSV-1 infections are generally associated with local mucocutaneous vesicular eruptions only. Dissemination of HSV occurs almost only in association with an underlying primary immune deficiency or secondary to immunosuppressive treatment. Disseminated infections can present with or without visible mucocutaneous lesions and may include sites that are rarely involved in an immunocompetent host, such as the lungs or the gastrointestinal tract. In virus-associated hemophagocytic syndrome, it is thought that the infection provokes an abnormal immune response, resulting in the secretion of cytokines, including γ-Interferon, IL18, and macrophage colony stimulating factor, by T-helper and dysfunctional cytotoxic T- and natural killer-cells. This results in massive macrophage activation with an associated macrophage-derived cytokine storm and phagocytosis of both erythrocytes and white blood cells [2, 10]. It is difficult to estimate if the inadequate immunologic response in our patient was a consequence of the pre-existing immune dysfunction (Wegener’s granulomatosis) or a result of the long-term immunosuppressive therapies.
Hemophagocytic syndrome may be difficult to distinguish from severe sepsis. Autopsy studies suggest that hemophagocytic syndrome may be underdiagnosed in intensive care patients [11]. It should be considered routinely in patients with unexplained multiple organ failure [12]. Diagnosis can be established when five of the eight following criteria are fulfilled: fever, splenomegaly, cytopenia of at least two cell lines, hypertriglyceridemia and/or hypofibrinogenemia, increased ferritin, increased soluble IL-2 receptor, decreased or absent natural killer-cell activity, hemophagocytosis in bone marrow, CSF, or lymph nodes [13].
The appropriate diagnostic approach to patients with suspected hemophagocytosis syndrome is not well defined. Potentially treatable causes of hemophagocytosis syndrome should be sought. Fisman [2] suggests that all patients with suspected infection-associated hemophagocytic syndrome should undergo extensive testing for underlying infections guided by epidemiological data and medical history. Chest radiography or CT scans, blood and urine cultures, screening for EBV, CMV and parvovirus B19, HIV, and human herpes virus 6, either through serologic testing or PCR should be performed. Additionally, fungal infections should be considered. Patients with a travel history should be screened for leishmaniasis, brucellosis, rickettsioses, and malaria. HSV screening has not been incorporated into guidelines yet. However, since it represents a treatable underlying cause, we recommend screening for HSV in all patients and, in particular, in immunosuppressed ones. As shown in this case report, HSV serology is not sufficient to rule out hemophagocytic syndrome caused by primary HSV-1 infection. Such cases are only detectable by positive PCR.
The therapy of infection-associated hemophagocytic syndrome has to weigh the benefit of attenuating excessive macrophage activity against the potential risk of an infection flare. The aim is to suppress the severe hyperinflammation responsible for the life-threatening symptoms. On the other hand, the pathogen must be eradicated, hence, removing the stimulus for the ongoing activation of T-cells. Pathogen control is usually not sufficient to control hyperinflammation. This was also demonstrated in our case; even if HSV-1 could be successfully suppressed in plasma by the use of acyclovir, the patient needed a second anti-inflammatory treatment with methylprednisolone to recover from the hemophagocytic syndrome. In patients with less severe symptoms, corticosteroids and immunoglobulins may be sufficient to control the excessive inflammation. However, if symptoms progress, the use of dexamethasone, etoposide, or cyclosporine A can be considered. In EBV-associated hemophagocytic syndrome for instance, etoposide has been shown to be effective [14].
In summary, hemophagocytic syndrome is a life-threatening condition, which may be difficult to diagnose. The reported case emphasizes the importance of an accurate diagnosis of potentially underlying infections because it influences the therapeutic approach. It is important to consider a variety of infectious agents, particularly in immunosuppressed individuals. We recommend to screen broadly for herpes viruses by serology and, perhaps even more importantly, by plasma DNA PCR.
References
Risdall RJ, McKenna RW, Nesbit ME, et al. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 1979; 44: 993–1002.
Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis 2000; 6: 601–8.
Pevenstein SR, Williams RK, McChesney D, et al. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol 1999; 73: 10514–8.
Fenaux P, Jouet JP, Zandecki M, et al. Hemophagocytic syndrome associated with herpes simplex virus. Apropos of a case with a fatal outcome. Nouv Rev Fr Hematol 1986; 28: 303–7.
Lasserre M, Huguet C, Terno O. Acute severe herpes simplex hepatitis with virus-associated hemophagocytic syndrome in an immunocompetent adult. J Hepatol 1993; 18: 256–7.
Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol 2005; 105: 1241–4.
Ramasamy K, Lim ZY, Savvas M, et al. Disseminated herpes virus (HSV-2) infection with rhabdomyolysis and hemophagocytic lymphohistiocytosis in a patient with bone marrow failure syndrome. Ann Hematol 2006; 85: 629–30.
Koizumi K, Yokoyama K, Nishio M, et al. A case of virus-associated hemophagocytic syndrome (VAHS) complicated by rhabdomyolysis which were associated with herpes-simplex virus infection. Rinsho Ketsueki 1996; 37: 40–5.
Wutzler P, Doerr HW, Färber I, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J Med Virol 2000; 61: 201–7.
Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome (HPS). Autoimmun Rev 2004; 3: 69–75.
Strauss R, Neureiter D, Westenburger B, et al. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients—a postmortem clinicopathologic analysis. Crit Care Med 2004; 32: 1316–21.
Stéphan F, Thiolière B, Verdy E, et al. Role of hemophagocytic histiocytosis in the etiology of thrombocytopenia in patients with sepsis syndrome or septic shock. Clin Infect Dis 1997; 25: 1159–64.
Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124–31.
Imashuku S, Kuriyama K, Teramura T, et al. Requirement for etoposide in the treatment of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol 2001; 19: 2665–73.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cusini, A., Günthard, H.F., Stussi, G. et al. Hemophagocytic syndrome caused by primary herpes simplex virus 1 infection: report of a first case. Infection 38, 423–426 (2010). https://doi.org/10.1007/s15010-010-0037-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-010-0037-9