Abstract
Background:
Currently, there is no apparent treatment for sarcopenia, which is characterized by diminished myoblast function. We aimed to manufacture exosomes that retain the myogenic differentiation capacity of human fetal cartilage-derived progenitor cells (hFCPCs) and investigate their muscle regenerative efficacy in myoblasts and a sarcopenia rat model.
Methods:
The muscle regeneration potential of exosomes (F-Exo) secreted during myogenic differentiation of hFCPCs was compared to human bone marrow mesenchymal stem cells-derived (hBMSCs) exosomes (B-Exo) in myoblasts and sarcopenia rat model. The effect of F-Exo was analyzed through known microRNAs (miRNAs) analysis. The mechanism of action of F-Exo was confirmed by measuring the expression of proteins involved in the Wnt signaling pathway.
Results:
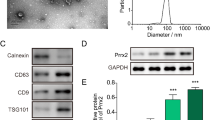
F-Exo and B-Exo showed similar exosome characteristics. However, F-Exo induced the expression of muscle markers (MyoD, MyoG, and MyHC) and myotube formation in myoblasts more effectively than B-Exo. Moreover, F-Exo induced greater increases in muscle fiber cross-sectional area and muscle mass compared to B-Exo in a sarcopenia rat. The miR-145-5p, relevant to muscle regeneration, was found in high concentrations in the F-Exo, and RNase pretreatment reduced the efficacy of exosomes. The effects of F-Exo on the expression of myogenic markers in myoblasts were paralleled by the miR-145-5p mimics, while the inhibitor partially negated this effect. F-Exo was involved in the Wnt signaling pathway by enhancing the expression of Wnt5a and β-catenin.
Conclusion:
F-Exo improved muscle regeneration by activating the Wnt signaling pathway via abundant miR-145-5p, mimicking the remarkable myogenic differentiation potential of hFCPCs.





Similar content being viewed by others
References
Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64.
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–17.
Lee KP, Shin YJ, Panda AC, Abdelmohsen K, Kim JY, Lee SM, et al. miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4. Genes Dev. 2015;29:1605–17.
Qazi TH, Duda GN, Ort MJ, Perka C, Geissler S, Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019;10:501–16.
Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53:e12857.
Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10:218.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Luo Y, Li Z, Wang X, Wang J, Duan X, Li R, et al. Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates. Front Bioeng Biotechnol. 2022;10:1016833.
Mas-Bargues C, Borras C. Importance of stem cell culture conditions for their derived extracellular vesicles therapeutic effect. Free Radic Biol Med. 2021;168:16–24.
Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:511.
Choi JS, Yoon HI, Lee KS, Choi YC, Yang SH, Kim IS, et al. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J Control Release. 2016;222:107–15.
Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transpl. 2016;25:449–61.
Kim J, Tran AN, Lee JY, Park SH, Park SR, Min BH, et al. Human fetal cartilage-derived progenitor cells exhibit anti-inflammatory effect on IL-1beta-mediated osteoarthritis phenotypes in vitro. Tissue Eng Regen Med. 2022;19:1237–50.
Lee SJ, Kim J, Choi WH, Park SR, Choi BH, Min BH. Immunophenotype and immune-modulatory activities of human fetal cartilage-derived progenitor cells. Cell Transpl. 2019;28:932–42.
Tran NT, Truong MD, Yun HW, Min BH. Potential of secretome of human fetal cartilage progenitor cells as disease modifying agent for osteoarthritis. Life Sci. 2023;324:121741.
Tran NT, Park IS, Truong MD, Park DY, Park SH, Min BH. Conditioned media derived from human fetal progenitor cells improves skin regeneration in burn wound healing. Cell Tissue Res. 2022;389:289–308.
Beier JP, Bitto FF, Lange C, Klumpp D, Arkudas A, Bleiziffer O, et al. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol Int. 2011;35:397–406.
Boscolo Sesillo F, Wong M, Cortez A, Alperin M. Isolation of muscle stem cells from rat skeletal muscles. Stem Cell Res. 2020;43:101684.
Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep. 2016;6:24436.
Wu Y, Deng W, Klinke DJ 2nd. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140:6631–42.
Shahini A, Choudhury D, Asmani M, Zhao R, Lei P, Andreadis ST. NANOG restores the impaired myogenic differentiation potential of skeletal myoblasts after multiple population doublings. Stem Cell Res. 2018;26:55–66.
Shin DI, Kim M, Park DY, Min BH, Yun HW, Chung JY, et al. Motorized shaver harvest results in similar cell yield and characteristics compared with rongeur biopsy during arthroscopic synovium-derived mesenchymal stem cell harvest. Arthroscopy. 2021;37:2873–82.
Nemoto A, Goyagi T. Tail suspension is useful as a sarcopenia model in rats. Lab Anim Res. 2021;37:7.
Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–32.
Wu S, Lin S, Zhang X, Alizada M, Wang L, Zheng Y, et al. Recent advances in cell-based and cell-free therapeutic approaches for sarcopenia. FASEB J. 2022;36:e22614.
Byun SE, Sim C, Chung Y, Kim HK, Park S, Kim DK, et al. Skeletal muscle regeneration by the exosomes of adipose tissue-derived mesenchymal stem cells. Curr Issues Mol Biol. 2021;43:1473–88.
Dey P, Soyer MA, Dey BK. MicroRNA-24-3p promotes skeletal muscle differentiation and regeneration by regulating HMGA1. Cell Mol Life Sci. 2022;79:170.
Wang B, Zhang A, Wang H, Klein JD, Tan L, Wang ZM, et al. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics. 2019;9:1864–77.
Cho KA, Choi DW, Kim YH, Kim J, Ryu KH, Woo SY. Mesenchymal stem cell-derived exosomes protect muscle loss by miR-145-5p activity targeting activin a receptors. Cells. 2021;10:2169.
Yin H, He H, Cao X, Shen X, Han S, Cui C, et al. MiR-148a-3p regulates skeletal muscle satellite cell differentiation and apoptosis via the PI3K/AKT signaling pathway by targeting Meox2. Front Genet. 2020;11:512.
Li Z, Liu C, Li S, Li T, Li Y, Wang N, et al. BMSC-derived exosomes inhibit dexamethasone-induced muscle atrophy via the miR-486-5p/FoxO1 axis. Front Endocrinol (Lausanne). 2021;12:681267.
Kim M, Shin DI, Choi BH, Min BH. Exosomes from IL-1beta-primed mesenchymal stem cells inhibited IL-1beta- and TNF-alpha-mediated inflammatory responses in osteoarthritic SW982 Cells. Tissue Eng Regen Med. 2021;18:525–36.
Liu B, Kong Y, Shi W, Kuss M, Liao K, Hu G, et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact Mater. 2022;14:61–75.
von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–9.
Du J, Li Q, Shen L, Lei H, Luo J, Liu Y, et al. miR-145a-5p Promotes myoblast differentiation. Biomed Res Int. 2016;2016:5276271.
Bernardi H, Gay S, Fedon Y, Vernus B, Bonnieu A, Bacou F. Wnt4 activates the canonical beta-catenin pathway and regulates negatively myostatin: functional implication in myogenesis. Am J Physiol Cell Physiol. 2011;300:C1122–38.
Han XH, Jin YR, Seto M, Yoon JK. A WNT/beta-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem. 2011;286:10649–59.
Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–52.
Armstrong DD, Wong VL, Esser KA. Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol. 2006;291:C185–8.
Cao H, Yue Z, Gao H, Chen C, Cui K, Zhang K, et al. In vivo real-time imaging of extracellular vesicles in liver regeneration via aggregation-induced emission luminogens. ACS Nano. 2019;13:3522–33.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI17C2191).
Author information
Authors and Affiliations
Contributions
The experiments were conceptualized and designed by DIS, JYJ, and BHM. The data collection and extraction were performed by DIS, JYJ and SJN. DIS, JYJ, HWY and DYP analyzed the data through productive discussions. DIS, JYJ and DYP were involved in writing and editing. BHM contributed to supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
The experiments were conducted with the approval of the Institutional Review Board (IRB) of Ajou University Medical Center (AJIRB-CRO-07–139) and the Institutional Animal Care and Use Committee (IACUC) in Ajou University (IACUC approval No. 2020–0017).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, D.I., Jin, Y.J., Noh, S. et al. Exosomes Secreted During Myogenic Differentiation of Human Fetal Cartilage-Derived Progenitor Cells Promote Skeletal Muscle Regeneration through miR-145-5p. Tissue Eng Regen Med 21, 487–497 (2024). https://doi.org/10.1007/s13770-023-00618-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00618-w