Abstract
Background:
Knee osteoarthritis (KOA) is a prevalent chronic joint disease caused by various factors. Mesenchymal stem cells (MSCs) therapy is an increasingly promising therapeutic option for osteoarthritis. However, the chronic inflammation of knee joint can severely impede the therapeutic effects of transplanted cells. Gelatin microspheres (GMs) are degradable biomaterial that have various porosities for cell adhesion and cell–cell interaction. Excellent elasticity and deformability of GMs make it an excellent injectable vehicle for cell delivery.
Methods:
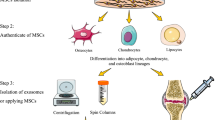
We created Wharton’s jelly derived mesenchymal stem cells (WJMSCs)-GMs complexes and assessed the effects of GMs on cell activity, proliferation and chondrogenesis. Then, WJMSCs loaded in GMs were transplanted in the joint of osteoarthritis mice. After four weeks, joint tissue was collected for histological analysis. Overexpressing-luciferase WJMSCs were performed to explore cell retention in mice.
Results:
In vitro experiments demonstrated that WJMSCs loaded with GMs maintained cell viability and proliferative potential. Moreover, GMs enhanced the chondrogenesis differentiation of WJMSCs while alleviated cell hypertrophy. In KOA mice model, transplantation of WJMSCs-GMs complexes promoted cartilage regeneration and cartilage matrix formation, contributing to the treatment of KOA. Compared with other groups, in WJMSCs+GMs group, there were fewer cartilage defects and with a more integrated tibia structure. Tracking results of stable-overexpressing luciferase WJMSCs demonstrated that GMs significantly extended the retention time of WJMSCs in knee joint cavity.
Conclusion:
Our results indicated that GMs facilitate WJMSCs mediated knee osteoarthritis healing in mice by promoting cartilage regeneration and prolonging cell retention. It might potentially provide an optimal strategy for the biomaterial-stem cell based therapy for knee osteoarthritis.





Similar content being viewed by others
References
Leilei D, Pengpeng Y, Haagsma JA, Ye J, Yuan W, Yuliang E, et al. The burden of injury in China, 1990–2017: findings from the Global Burden of Disease Study 2017. Lancet Public Health. 2019;4:e449–61.
Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819–28.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59.
Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41.
Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96:624–30.
Makarczyk MJ, Gao Q, He Y, Li Z, Gold MS, Hochberg MC, et al. Current models for development of disease-modifying osteoarthritis drugs. Tissue Eng Part C Methods. 2021;27:124–38.
Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–62.
Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19:24.
Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597–606.
Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695–704.
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transpl. 2014;23:1045–59.
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90.
Mitrano TI, Grob MS, Carrión F, Nova-Lamperti E, Luz PA, Fierro FS, et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–25.
Klontzas ME, Kenanidis EI, Heliotis M, Tsiridis E. Mantalaris A. Bone and cartilage regeneration with the use of umbilical cord mesenchymal stem cells. Expert Opin Biol Ther. 2015;15:1541–52.
Xiang XN, Zhu SY, He HC, Yu X, Xu Y, He CQ. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022;13:14.
Van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–96.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–53.
Huang J, Liu Q, Xia J, Chen X, Xiong J, Yang L, et al. Modification of mesenchymal stem cells for cartilage-targeted therapy. J Transl Med. 2022;20:515.
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24.
Kim H, Bae C, Kook YM, Koh WG, Lee K, Park MH. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res Ther. 2019;10:51.
Zonderland J, Moroni L. Steering cell behavior through mechanobiology in 3D: a regenerative medicine perspective. Biomaterials. 2021;268:120572.
Thorp H, Kim K, Kondo M, Maak T, Grainger DW, Okano T. Trends in articular cartilage tissue engineering: 3D mesenchymal stem cell sheets as candidates for engineered hyaline-like cartilage. Cells. 2021;10:643.
Saraswat R, Ratnayake I, Perez EC, Schutz WM, Zhu Z, Ahrenkiel SP, et al. Micropatterned biphasic nanocomposite platform for maintaining chondrocyte morphology. ACS Appl Mater Interfaces. 2020;12:14814–24.
Glowacki J, Trepman E, Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med. 1983;172:93–8.
Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, et al. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008;14:667–80.
Li YY, Choy TH, Ho FC, Chan PB. Scaffold composition affects cytoskeleton organization, cell-matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation. Biomaterials. 2015;52:208–20.
Li Y, Liu W, Liu F, Zeng Y, Zuo S, Feng S, et al. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc Natl Acad Sci U S A. 2014;111:13511–6.
Van Der Sluijs JA, Geesink RG, Van Der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10:58–61.
Rutgers M, Van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:12–23.
Pinamont WJ, Yoshioka NK, Young GM, Karuppagounder V, Carlson EL, Ahmad A, et al. Standardized histomorphometric evaluation of osteoarthritis in a surgical mouse model. J Vis Exp. 2020;159:e60991.
Jiao Y, Chen X, Niu Y, Huang S, Wang J, Luo M, et al. Wharton’s jelly mesenchymal stem cells embedded in PF-127 hydrogel plus sodium ascorbyl phosphate combination promote diabetic wound healing in type 2 diabetic rat. Stem Cell Res Ther. 2021;12:559.
Doege KJ, Garrison K, Coulter SN, Yamada Y. The structure of the rat aggrecan gene and preliminary characterization of its promoter. J Biol Chem. 1994;269:29232–40.
Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016;17:1164.
Swanson WB, Omi M, Zhang Z, Nam HK, Jung Y, Wang G, et al. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials. 2021;272:120769.
Gupte MJ, Swanson WB, Hu J, Jin X, Ma H, Zhang Z, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1–11.
Corradetti B, Taraballi F, Minardi S, Van Eps J, Cabrera F, Francis LW, et al. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl Med. 2016;5:670–82.
Chang YH, Wu KC, Wang CC, Ding DC. Enhanced chondrogenesis of human umbilical cord mesenchymal stem cells in a gelatin honeycomb scaffold. J Biomed Mater Res A. 2020;108:2069–79.
Ke W, Ma L, Wang B, Song Y, Luo R, Li G, et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022;150:83–95.
Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–102.
Jahangir S, Eglin D, Pötter N, Khozaei Ravari M, Stoddart MJ, Samadikuchaksaraei A, et al. Inhibition of hypertrophy and improving chondrocyte differentiation by MMP-13 inhibitor small molecule encapsulated in alginate-chondroitin sulfate-platelet lysate hydrogel. Stem Cell Res Ther. 2020;11:436.
Rajagopal K, Ramesh S, Madhuri V. Early addition of parathyroid hormone-related peptide regulates the hypertrophic differentiation of mesenchymal stem cells. Cartilage. 2021;13:143S–52S.
Tang Z, Lu Y, Zhang S, Wang J, Wang Q, Xiao Y, et al. Chondrocyte secretome enriched microparticles encapsulated with the chondrocyte membrane to facilitate the chondrogenesis of BMSCs and reduce hypertrophy. J Mater Chem B. 2021;9:9989–10002.
Xie M, Zhang Y, Xiong Z, Hines S, Shang J, Clark KL, et al. Generation of hyaline-like cartilage tissue from human mesenchymal stromal cells within the self-generated extracellular matrix. Acta Biomater. 2022;149:150–66.
Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res. 2019;37:1229–35.
Liu W, Li Y, Zeng Y, Zhang X, Wang J, Xie L, et al. Microcryogels as injectable 3-D cellular microniches for site-directed and augmented cell delivery. Acta Biomater. 2014;10:1864–75.
Jiao Y, Chen X, Nong B, Luo M, Niu Y, Huang S, et al. Transplantation of Wharton’s jelly mesenchymal stem cells encapsulated with Hydroactive® Gel promotes diabetic wound antifibrotic healing in type 2 diabetic rats. J Mater Chem B. 2022;10:8330–46.
Zhang X, Liu S, Wang Z, Luo C, Dai Z, Sun J, et al. Implanted 3D gelatin microcryogel enables low-dose cell therapy for osteoarthritis by preserving the viability and function of umbilical cord MSCs. Chem Eng J. 2021;416:129140.
De Lange-Brokaar BJ, Ioan-Facsinay A, Van Osch GJ, Zuurmond AM, Schoones J, Toes RE, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–99.
Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–300.
Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, Van Den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–57.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31971365 and 82271688) and the Guangdong Basic and Applied Basic Research Foundation (2020B1515120090)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
The animal studies were performed after receiving approval the Institutional Animal Care and Use Committee (IACUC) in Sun Yat-sen University, P.R. China. (IACUC approval No. SYSU-IACUC-2021-B1256).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Huang, S., Niu, Y. et al. Transplantation of Gelatin Microspheres Loaded with Wharton's Jelly Derived Mesenchymal Stem Cells Facilitates Cartilage Repair in Mice. Tissue Eng Regen Med 21, 171–183 (2024). https://doi.org/10.1007/s13770-023-00574-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00574-5