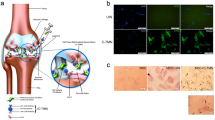
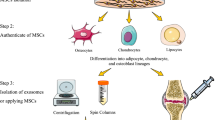
Abstract
Osteoarthritis (OA) is a chronic degenerative joint disease characterized by the destruction of the articular cartilage, sclerosis of the subchondral bone, and joint dysfunction. Its pathogenesis is attributed to direct damage and mechanical destruction of joint tissues. Mesenchymal stem cells (MSCs), suggested as a potential strategy for the treatment of OA, have shown therapeutic effects on OA. However, the specific fate of MSCs after intraarticular injection, including cell attachment, proliferation, differentiation, and death, is still unclear, and there is no guarantee that stem cells can be retained in the cartilage tissue to enact repair. Direct homing of MSCs is an important determinant of the efficacy of MSC-based cartilage repair. Recent studies have revealed that the unique homing capacity of MSCs and targeted modification can improve their ability to promote tissue regeneration. Here, we comprehensively review the homing effect of stem cells in joints and highlight progress toward the targeted modification of MSCs. In the future, developments of this targeting system that accelerate tissue regeneration will benefit targeted tissue repair.
Graphical Abstract

Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a chronic joint disease characterized by articular cartilage (AC) degeneration and subchondral bone hyperplasia. OA is one of the leading causes of disability in the elderly and during adulthood. As the elderly population increases over the coming decades, the prevalence of arthritis will also increase. Currently, treatment options for OA are limited to pain relief and joint replacement surgery [1]. Targeted repair of the damaged cartilage and restoration of joint function is critical to treating osteoarthritis. Cell therapy, especially transplantation of mesenchymal stem cells/stromal cells (MSCs), represents an effective solution for tissue regeneration and repair as these pluripotent stem cells have the potential to differentiate into cartilage. MSCs can be used as seed cells that directly participate in the local repair and regulate metabolism and immune function through their secretory functions, thereby preventing or delaying the need for joint replacement surgery. Given their versatility, MSCs may play key roles in different stages of cartilage repair. Moreover, because the joint cavity is a relatively closed space and can be easily targeted by injection, the feasibility of injecting MSCs to treat joint diseases is significant compared to treating diseases that require systemic MSC injection. Several pre-clinical and clinical studies of MSC therapy in OA have indicated its safety and reliability [2,3,4]. However, the ability to effectively deliver exogenous MSCs to the injury site for enhancing the regeneration process remains an outstanding challenge for cell therapy. Although intra-articular (IA) injection of ex vivo expanded MSCs can increase the number of cells in the joint cavity, most MSCs fail to migrate toward the injured area. Due to the low targeting efficiency of MSCs, current MSC-based regenerative therapies require IA injection of a large number of cells.
Advances in biology, genetic engineering, and chemical biology related to MSCs have opened up new avenues for enhancing the efficacy of MSCs in treating OA. Targeted modification of MSCs can enhance their migration potential and facilitate their homing ability, translating preclinical research into effective and safe targeted therapies.
Here, we review recent advances in targeted cell therapy to repair of articular cartilage (AC) tissue in OA, with special attention to AC. We describe the structure and function of healthy and transparent AC and OA and current strategies for enhancing the delivery of MSCs to cartilage tissue. We focus primarily on targeting methods, including cell surface modifications and magnetic-assisted tissue targeting, discuss their advantages and limitations, provide additional perspectives, and examine emerging strategies based on new research findings. These findings are being verified in preclinical models, which are expected to develop into early proof-of-concept trials and provide information for designing future definitive clinical trials.
Stem cells in AC repair
Unlike tissues with vascular blood supply, avascular cartilage tissue does not immediately trigger an inflammatory response upon injury, limiting its ability to promote repair and self-regeneration [5]. Therefore, endogenous cells may be recruited to diseased sites and act as key players in tissue regeneration. Stem cells (progenitor cells) with self-renewal capacity, identified in the surface regions of AC, have been designated cartilage-derived stem/progenitor cells [6]. Synovial fluid/synovial membrane MSCs have also been found to support self-repair in the joint cavity [7]. The potential applications of these cells remain unclear, but ongoing research seeks to better understand these cellular phenotypes and their therapeutic value for cartilage repair.
MSCs have the capacity for self-renewal and chondrogenic differentiation, making them an optimal cellular source for cartilage regeneration. MSCs can be derived from a variety of autologous tissues, including bone marrow (BMSCs), adipose tissue (ADSCs), synovial tissue (SDSCs), and peripheral blood (PB-MSCs) [8, 9]. Based on the specific cartilage pathology, MSCs can be implanted into the defect area after a surgical incision or administered by IA injection.
In 2008, Centeno et al. first reported the injection of autologous BMSCs in patients with degenerative cartilage disease [10]. Preliminary clinical data from Qiao et al. [59] showed no adverse events and significant therapeutic benefits after the highest dose of ADSC injection. Furthermore, the combined effect of the scaffold material has become more obvious. Hallam and colleagues seeded BMSCs with a platelet fibrin glue (FG) scaffold and demonstrated significant cartilage renewal by clinical MRI [11]. Similarly, Kuroda and coworkers implanted BMSCs onto collagen membranes, and reported a significant improvement in defects filled with hyaline-like cartilage tissue [12].
We have summarized representative current clinical uses of MSC transplantation for the treatment of cartilage injury and OA in Table 1. Regardless of cell source or implantation method, most studies corroborate the clinical benefits of MSCs in AC regeneration. However, the utility of MSCs remains debatable due to many unanswered questions. According to the International Association for Cartilage Repair criteria, 76% of patients who receive MSC implantation exhibited abnormal or severely repaired tissue upon second-look arthroscopic assessment [13]. Thus, reliable clinical confirmation of the safety and efficacy of this approach is required through double-blind, controlled, prospective multicenter studies with longer follow-up duration. Indeed, intra-articular injection of MSCs must be performed directly into the injury site to receive a therapeutic benefit. However, lack of targeting may lead to cell diffusion into non-target tissues, posing a potential barrier to the clinical translation of MCS-based cartilage therapies. Off-target effects appear to cause low engraftment. For example, in a rabbit model, MSCs were found to migrate to the upper knee, subchondral bone, and popliteal fossa following IA injection, but no MSCs were seeded in the cartilage defects [14]. Therefore, improved tracking of transplanted cells in the cartilage is needed to better understand the mechanisms underlying MSC migration and homing. The targeted engineering of MSCs promises to further improve clinical outcomes for local cartilage lesions.
BMSCs, bone-marrow-derived MSCs; ADSCs, adipose-derived stem cells; hUC-MSCs, human umbilical-cord-derived MSCs; hUCB-MSCs, human umbilical cord blood-derived MSCs; MRI, magnetic resonance imaging; VAS, visual analog scale; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; KSS, Knee Society Score; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; OA, osteoarthritis; IA, intra-articular.
Mesenchymal stromal cell homing
When tissues and organs are injured, natural repair mechanisms are activated to release MSCs into circulation that migrate to the damaged tissue sites and secrete powerful immunomodulatory, angiogenic, and anti-apoptotic factors to create a regeneration-promoting microenvironment [45,46,47,48]. For cartilage defects, including OA, the tissue has been reported to regenerate via homing of endogenous cells. Synovial tissue can potentially recruit endogenous stem cells, which facilitate partial tissue regeneration even in the absence of exogenous cell transplantation.
Exogenously transplanted MSCs also tend to migrate into tissues and affect tissue regeneration. The use of exogenously transplanted stem cells as "biological regeneration supplements" is largely based on their natural abilities to mobilize, migrate, and home. Mechanisms of cell migration and “nesting” into injury sites mediated by a broad range of chemokine and growth factor receptors are primarily relevant to MSCs [49, 50]. The most well-studied examples include stromal cell-derived factor 1 (SDF1) and its receptor and CXC-chemokine receptor 4 (CXCR4) implicated in MSC homing [51]. SDF-1 has been shown to be upregulated at injury sites and to affect MSC migration in a dose-dependent manner. Therefore, SDF-1 has been used to induce migration and homing of MSCs to cartilage defect sites for enhancing tissue repair [52, 53]. Other chemokine-chemokine receptor pairs, including PDGF-PDGFR, SCF-c-Kit, HGF-c-Met, VEGF-VEGFR, MCP-1-CCR2 and HMGB1-RAGE, are also involved in MSC recruitment and migration [54]. Thus, MSCs can be modulated for therapeutic purposes,, and external cues can enhance their homing efficiency towards damaged tissues.
Pretreatment of MSCs with specific compounds, cytokines, and hypoxic conditions can enhance cell migration toward the injury site. Increased expression of the cytokine membrane receptor (CXCR4) can be induced by stimulation with Fms-like tyrosine kinase (Flt-3) ligands, stem cell factor (SCF), interleukin (IL), or hepatocyte growth factor (HGF) [55]. Preconditioning of MSCs with tumor necrosis factor alpha (TNFα) can improve the migration of MSCs to the site of injury and affect osteoclast function [56]. Similarly, MSC preconditioning with insulin growth factor-1 (IGF-1) has been shown to increase the expression of CXCR4 and improve cell migration capacity in vitro and in vivo [41,[57]. Other small molecules, such as glycogen synthase kinase-3β inhibitors, lithium, and the histone deacetylase inhibitor valproate, can effectively enhance MSC migration ability by upregulating the expression of CXCR4 and matrix metalloproteinases (MMP) [58] (See Fig. 1).
Another strategy to improve MSC homing ability is to use genetic manipulation to increase the expression of targeted molecules. Many research groups have reported that CXCR4 overexpression exhibits variable efficiency in increasing the targeting potential of MSCs.
Engineered MSCs for targeted therapy
Although endogenous homing mechanisms help MSCs reach and engraft at the target site, most MSCs fail to attach to the damaged cartilage layer. The fate of MSCs following intra-articular delivery is still unclear due to the vigorous metabolism of synovial fluid in the joint cavity. It is possible that after MSCs are injected into the joint cavity, they quickly spread into systemic circulation due to the rapid turnover of synovial capillaries and lymphatic vessels, resulting in only the transient presence of MSCs in the joint cavity. Moreover, the abundance of anionic proteoglycans in the cartilage layer endows the tissue matrix with high density and a high negative fixed charge density, making it extremely challenging to retain MSCs in the cartilage tissue [59]. As illustrated in Fig. 2, the multi-zonal structure of AC makes it quite difficult for MSCs to penetrate through the cartilage surface to reach the tissue zones.
Morphology of the cartilage tissue. The superficial zone consists of a high concentration of collagen fibers parallel to the articular surface. This layer is rich in type II collagen and contains small amounts of type I collagen and proteoglycans. The middle zone is the thickest cartilage layer and accounts for 40–60% of articular cartilage volume. Collagen fibers in this area are thicker and contain higher levels of proteoglycans. The deep region has the highest concentration of proteoglycans where chondrocytes and collagen fibers are arranged in vertical columns perpendicular to the surface.. In areas of calcification, a proteoglycan-free matrix surrounds round chondrocytes with hypertrophic phenotypes
MSCs can be genetically modified virally and non-virally to overexpress therapeutic proteins and targeting moieties. In addition, chemical conjugation, non-covalent interactions, and enzymatic modifications have been used to coat MSC membranes with targeting groups (Fig. 3). MSCs can also be treated with non-peptide drugs or magnetic nanoparticles to enhance their efficacy and targeted delivery.
Strategies for the targeted delivery of MSCs to articular cartilage. IA of MSCs showed both tissue regeneration potential and paracrine effects (e.g., anti-apoptosis, reduced inflammation, or regulation of immune responses). MSC surfaces can be conjugated with specific antibodies against antigens present on the cartilage surface (e.g., anti- cartilage matrix proteoglycan, Col II) (Abs). Surface modification of MSCs via cartilage matrix-targeting peptides imparts additional functionality to enable site-specific delivery. Genetically engineered MSCs highly expressing CXCR4 facilitate MSC recruitment to the cartilage via a homing mechanism. In addition, magnetic targeting systems can direct the delivery of MSCs to the desired region by using external magnetic forces
Genetically modified MSCs for targeted therapy
Overexpression of chemokine receptors such as CXCR1, CXCR4, or CXCR7 has been shown to enhance the migration and targeting ability of MSCs [60]. Cho, et al. claimed that intravenous infusion of autologous MSCs overexpressing CXCR4 significantly inhibited bone loss in an OVX-induced mouse model. Furthermore, MSCs overexpressing RANK-Fc effectively enhance bone-protective effects [61]. In a mouse model of myocardial infarction, increased CXCR4 expression can induce migration toward the infarction site, improving cardiac performance [62]. These studies indicated the value of enhancing CXCR4 expression to regulate MSC trafficking.
While non-viral methods are preferred, particularly in the context of potential clinical applications, they remain limited due to their low transfection efficiency. Several cationic liposomal reagents (such as IBAfect, a polycationic liposomal transfection reagent) have been used to achieve superior CXCR4 transfection efficiency [63].
Surface engineering of MSCs by antibodies for targeted therapy
Biomedical engineering offers new opportunities for surface modification of living cells with antibodies. Various antibodies can be applied to cell surfaces as uniform ultrathin coatings via hydrophobic interactions, covalent binding, or lipid-PEG methodology. After functionalization with antibody conjugation, the cell surface can bind to a specific antigen on the target tissue.
In the lipidation method, palmitate-conjugated protein A/G is bound to the Fc region of the Ab [58]. Palmitate-derivatized antibodies against vascular adhesion molecules ICAM-1, VCAM-1 and MAdCAM-1 have been shown to enhance the homing of surface-engineered cells [64,65,66]. Dennis et al. embedded lipidated protein G into the membranes of chondrogenic progenitor cells, allowing subsequent binding of anchor protein G antibodies to cartilage matrix antigens on the extracellular surface. Cells coated with multiple antibodies were found to preferentially adhere to cartilage repair sites when added to rabbit cartilage explants [67]. Modifying the cell membrane with palmitate-conjugated type II collagen enables efficient targeted delivery of therapeutic MSCs to the osteochondral defect explant [64]. These results suggest that coating cell membranes with antibodies against matrix molecules effectively promotes the adhesion of MSCs to specific cartilage damage locations.
Peptide functionalization of MSCs for targeted modification
Cell-homing peptides (CHPs) are highly specific affinity peptides that target the cell surface. Several research groups have exploited CHPs for cartilage treatment. Previously, a self-assembling peptide (SAP) functionalized with the bone marrow homing peptide (BMHP) motif SKPPGTSS was designed to regulate MSC homing and promote the repair of cartilage defects after microfracture [68].
Pi et al. identified a short CHP sequence with a high binding affinity toward chondrocytes. They introduced a non-viral vector in which fluorescently labeled chondrocyte-affinity peptide (CAP) was covalently bound to polyethyleneimine (PEI) and injected into rabbit knees to target hyaline cartilage. The results using fluorescein isothiocyanate (FITC)-labeled CAP-PEI entered into the chondrocytes. demonstrating cartilage-specific targeting [69].
In a recent report, we also demonstrated that a CAP-modified exosome could deliver drugs to chondrocytes in joints, alleviating OA in a rat model [70, 71]. Similarly, Cheung et al. used phage display screening technology to identify two cartilage-binding peptide sequences of 12 amino acids in length that specifically bound cartilage ECM and chondrocytes, showing that these polypeptides adhered strongly to the surface of chondrocytes [72]. Recently, Sangar. et al. identified a cystine-dense peptide (CDP) that rapidly accumulated in the cartilage after systemic injection. The accumulating peptide CDP-11R reached the articular cartilage layer within 30 min and wa detectable for more than 4 days [73].
A domain in placental growth factor-2 (PlGF-2(123-144)) was found to bind ECM proteins with high affinity [74]. As the cartilage tissue is rich in ECM proteins, using engineered TNF α conjugated with the PlGF-2123-144 peptide could enhance local retention time in the cartilage [75]. Similarly, a recent study by Delint et al. had rationally designed a nanocomplex composed of PlGF-2 fused to the supercharged green fluorescent protein (scGFP). The complex was then electrostatically coupled to anionic polymer surfactant chains to generate oxidized poly-oxyethylene non-ylphenyl ether (S-) scGFP_PIGF2 nanocomplexes, which were spontaneously inserted into the plasma membrane of hMSCs. Their findings indicated that PIGF nanocomplex-modified hMSCs had significantly increased affinity for collagen II, a cartilage ECM component, and high concentrations of hMSCs were detected at the cartilage interface [76]. Thus, modification of hMSC membranes with scGFP_PlGF2 can improve the efficacy of stem cell-based injection therapies for damaged articular cartilage.
Bifunctional peptide-modified functional ferritin is another example developed to promote BMSC engraftment for cartilage regeneration. Researchers engineered ferritin nanocages containing RGD peptides that could target BMSCs and WYRGRL peptides with intrinsic affinity for the cartilage matrix component of collagen II [77]. The combination of these two significant peptides enabled the recruitment of exogenous MSCs to areas of defective cartilage. In Table 2, we have listed all ligands that can be used for cartilage-targeted MSC delivery.
The advantages of CHP include high targeting specificity, ease of synthesis, small size, low molecular weight, and high biocompatibility. Attaching multiple ligands simultaneously to the cell surface or other carriers is possible. The unique ability of CHP to target specific tissues makes them promising candidates for cellular delivery in clinical settings. Especially for AC, chondrocyte-homing peptides can be integrated onto the surface of MSCs to deliver therapeutic MSCs throughout diseased tissues. Although MSC surface engineering approaches have great therapeutic potential, they may alter MSC membrane properties. Also, the associated biosafety issues limit their clinical applications. For example, covalent anchoring of peptides or Abs to the MSC surface may interfere with membrane protein function and affect signaling pathways, resulting in aberrant ligand-receptor binding and may alter cell fate.
Magnetic stem cell targeting
For magnetic MSC delivery, magnetically loaded cells are administered to target areas with the assistance of a magnetic field. MSCs typically internalize nanoparticles by passive diffusion or endocytosis upon adding magnetic nanoparticles (MNPs) to the cell culture medium. Some commonly used MNPs, such as nickel and cobalt, may be somewhat toxic to the cells and for in vivo applications. However, iron oxide magnetite (Fe3O4) and maghemite (γ-Fe2O3) have been identified as biocompatible MNPs. Compared to magnetite MNPs, maghemite MNPs cause less damage to recipient cells due to the oxidized state of iron (Fe3+). In preclinical studies, magnetic stem cell targeting has been used to concentrate MSCs in bone or cartilage tissue [89].
We have developed magnetic nanocomposite-combined MSCs for the treatment of cartilage defects. Stem cell differentiation was promoted by exposure to a pulsed electromagnetic field, which has broad applications in cartilage tissue engineering [90,91,92]. Kobayashi et al. labeled BMSCs with Feridex and injected them into rabbit and pig models of osteochondral defect, showing enhanced engraftment into the chondral defect under external magnetic force [93]. It was demonstrated that besides improved MSC proliferation due to magnetic labeling with ferucarbotran, targeted delivery of MSCs to the injury site using an external magnetic device resulted in complete repair and integration of the targeted tissue. Thus, MSC delivery using a magnetic targeting system has the potential to overcome barriers inhibiting the repair of severe chronic osteochondral defects. Furthermore, delivery of magnetically labeled MSCs to target tissues allows their retention in the cartilage defect area long enough to repair full-thickness cartilage defects in a mini-pig model [94].
A clinical study evaluated the safety and efficacy of magnetic targeting of MSCs in patients with focal cartilage defects in the knee joint. Autologous bone marrow MSCs were magnetized with ferucarbotran and injected into the knee joint in the presence of a 1.0 Tesla (T) magnetic force. No serious adverse events were observed during magnet-targeted therapy. After 48 weeks of treatment, MRI showed that the cartilage defect area was almost completely filled with cartilage-like tissue [95]. These findings suggest that magnetic targeting of MSCs is safe and significantly improves clinical outcomes and, therefore can be used as a minimally invasive treatment for cartilage repair.
MNPs that are sufficiently small (between 10 and 30 nm) can exhibit superparamagnetic behavior, and such superparamagnetic nanoparticles (SPIONs) are important materials for potential clinical applications of enhanced MSC-based cell therapy. Furthermore, they can be used for MSC labeling and as in vivo tracking agents due to the strong signals they generate under MRI. For example, SPION-ASC-labeled ASCs were successfully tracked by MRI following injection into the knee joint. The implanted ASCs adhered to the injured meniscus and differentiated into meniscus tissue under the action of a permanent external magnet [96].
In recent years, the concept of a magnetic microrobot has been proposed. Under the action of a magnetic field, magnetically driven microrobot-targeted cell delivery could significantly improve the low targeting efficiency of MSCs to promote tissue regeneration [97]. A microrobot loaded with human adipose-derived MSCs was guided by a magnetic field to specific lesions in rabbit knee cartilage to stimulate regeneration. The microrobot degraded within three weeks without causing inflammation in rabbits, indicating good biocompatibility and biodegradability [98]. The applications of magnetically targeted MSCs to animal models and clinical studies are summarized in Table 3.
Conclusions and perspectives
MSCs have been widely used in cartilage repair due to their self-renewing pluripotency and differentiation ability. Over the past few decades, MSC-based therapies have emerged as promising new therapeutics in regenerative medicine. While the results of clinical studies have been very positive, some inconsistent data have emerged from Phase I/II trials. Intra-articular injection of MSCs results in limited cell retention and survival in the cartilage. Therefore, the cartilage regeneration capacity of exogenous MSCs following transplantation is limited. Modification strategies can be combined with compounds that enhance MSC survival, migration, homing, and adhesion to optimize cell survival and maximize therapeutic efficacy. Also, the route of administration, number of modified cells administered, and engraftment frequency require further improvement.
In evaluating the fate and efficacy of MSCs, innovative in vivo imaging strategies and quantitative assays are critical to determining MSC distribution, viability, and function. In addition, using appropriate ex vivo cartilage and animal models can provide further insight into pharmacokinetics and pharmacodynamics under specific pathological conditions. Overall, cell-based targeted therapies represent a major new development direction for accelerating the clinical translation of MSCs to treat cartilage diseases.
Availability of data and materials
Not applicable.
Abbreviations
- OA:
-
Osteoarthritis
- MSC:
-
Mesenchymal stem cells
- IA:
-
Intra-articular
- AC:
-
Articular cartilage
- CPSCs:
-
Cartilage-derived stem/progenitor cells
- UC-MSCs:
-
Umbilical cord MSCs
- BMSCs:
-
Bone marrow-derived MSCs
- ADSCs:
-
Adipose tissue-derived stem cells
- SDSCs:
-
Synovial tissue-derived stem cells
- PB-MSCs:
-
Peripheral blood-derived MSCs
- hADMSCs:
-
Human adipose-derived MSCs
- SPIONs:
-
Superparamagnetic nanoparticles
- PRP:
-
Platelet-rich plasma
- SDF1:
-
Cell-derived factor 1
- CXCR4:
-
CXC-chemokine receptor 4
- TNFα:
-
Tumor necrosis factor alpha
- IGF-1:
-
Insulin growth factor-1
- MMP:
-
Matrix metalloproteinases
- MNPs:
-
Magnetic nanoparticles
- CHPs:
-
Cell homing peptides
- GFP:
-
Green fluorescent protein
- CDP:
-
cystine-dense peptide
- CPC:
-
Cartilage penetrating cationic peptide
- CAP:
-
Chondrocyte-affinity peptide
References
DeRogatis M, Anis HK, Sodhi N, Ehiorobo JO, Chughtai M, Bhave A, Mont MA. Non-operative treatment options for knee osteoarthritis. AnnTranslat Med. 2019;7:S245–S245.
Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545.
Emadedin M, Labibzadeh N, Liastani MG, Karimi A, Jaroughi N, Bolurieh T, Hosseini S-E, Baharvand H, Aghdami N. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20:1238–46.
Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–8.
Chiang H, Jiang C-C. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009;108:87–101.
Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11:206–12.
de Sousa EB, Casado PL, Moura Neto V, Duarte MEL, Aguiar DP. Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014;5:112–112.
Perdisa F, Gostyńska N, Roffi A, Filardo G, Marcacci M, Kon E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cells International. 2015;2015: 597652.
Reissis D, Tang QO, Cooper NC, Carasco CF, Gamie Z, Mantalaris A, Tsiridis E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther. 2016;16:535–57.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Phys 2008;11(3):343–53.
Haleem AM, Singergy AAE, Sabry D, Atta HM, Rashed LA, Chu CR, El Shewy MT, Azzam A, Abdel Aziz MT. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–61.
Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42:1628–37.
Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, Tan HB. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75:432–8.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–53.
Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García-Sancho J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95:1535–41.
Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston A, Chiovitti J, Keating A, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. 2019;8:746–57.
Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis preliminary report of four patients. Int J Rheum Dis. 2011;14:211–5.
Emadedin M, Labibzadeh N, Liastani MG, Karimi A, Jaroughi N, Bolurieh T, Hosseini SE, Baharvand H, Aghdami N. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20:1238–46.
Al-Najar M, Khalil H, Al-Ajlouni J, Al-Antary E, Hamdan M, Rahmeh R, Alhattab D, Samara O, Yasin M, Abdullah AA, et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 2017;12:190.
Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246.
Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Núñez-Córdoba JM, López-Elío S, Andreu E, Sánchez-Guijo F, Aquerreta JD, Bondía JM, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2018;16:213.
Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219–25.
Emadedin M, Ghorbani Liastani M, Fazeli R, Mohseni F, Moghadasali R, Mardpour S, Hosseini SE, Niknejadi M, Moeininia F, Aghahossein Fanni A, et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med. 2015;18:336–44.
de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MP, Nizak R, van Rijen MH, de Weger RA, Dhert WJ, Saris DB. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35:256–64.
Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301.
Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–90.
Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295–307.
Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med. 2015;43:2738–46.
Kim YS, Choi YJ, Lee SW, Kwon OR, Suh DS, Heo DB, Koh YG. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthr Cartilage. 2016;24:237–45.
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5:847–56.
Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J. 2016;36:229–36.
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–66.
Freitag J, Wickham J, Shah K, Tenen A. Real-world evidence of mesenchymal stem cell therapy in knee osteoarthritis: a large prospective two-year case series. Regen Med. 2022;17(6):355–73.
Lee NH, Na SM, Ahn HW, Kang JK, Seon JK, Song EK. Allogenic human umbilical cord blood-derived mesenchymal stem cells are more effective than bone marrow aspiration concentrate for cartilage regeneration after high tibial osteotomy in medial unicompartmental osteoarthritis of knee. Arthroscopy. 2021;37:2521–30.
Lim H-C, Park Y-B, Ha C-W, Cole BJ, Lee B-K, Jeong H-J, Kim M-K, Bin S-I, Choi C-H, Choi CH, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop J Sports Med. 2021;9:2325967120973052.
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8:215–24.
Dilogo IH, Canintika AF, Hanitya AL, Pawitan JA, Liem IK, Pandelaki J. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol. 2020;30:799–807.
Park Y-B, Ha C-W, Lee C-H, Yoon YC, Park Y-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Song J-S, Hong K-T, Kim N-M, Jung J-Y, Park H-S, Lee SH, Cho YJ, Kim SJ. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regenerative therapy. 2020;14:32–9.
Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902–7.
Sadlik B, Jaroslawski G, Gladysz D, Puszkarz M, Markowska M, Pawelec K, Boruczkowski D, Oldak T. Knee cartilage regeneration with umbilical cord mesenchymal stem cells embedded in collagen scaffold using dry arthroscopy technique. Adv Exp Med Biol. 2017;1020:113–22.
Khalifeh Soltani S, Forogh B, Ahmadbeigi N, Hadizadeh Kharazi H, Fallahzadeh K, Kashani L, Karami M, Kheyrollah Y, Vasei M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21:54–63.
Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473:2316–26.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–38.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96.
Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181–96.
Ullah M, Liu DD, Thakor AS. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15:421–38.
Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–46.
Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45.
Xiang X, Liu H, Wang L, Zhu B, Ma L, Du F, Li L, Qiu L. Ultrasound combined with SDF-1α chemotactic microbubbles promotes stem cell homing in an osteoarthritis model. J Cell Mol Med. 2020;24:10816–29.
Sukegawa A, Iwasaki N, Kasahara Y, Onodera T, Igarashi T, Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 2012;18:934–45.
Zhang F, Leong W, Su K, Fang Y, Wang DA. A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng Part A. 2013;19:1091–9.
Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology. 2014;54:210–8.
Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904.
Goodman SB, Lin T. Modifying MSC phenotype to facilitate bone healing: biological approaches. Front Bioeng Biotechnol. 2020;8:641–641.
Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–4.
Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–9.
Vedadghavami A, Zhang C, Bajpayee AG. Overcoming negatively charged tissue barriers: drug delivery using cationic peptides and proteins. Nano Today. 2020;34: 100898.
Nowakowski A, Walczak P, Lukomska B, Janowski M. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cells Int. 2016;2016:4956063.
Cho SW, Sun HJ, Yang JY, Jung JY, An JH, Cho HY, Choi HJ, Kim SW, Kim SY, Kim D, Shin CS. Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol Ther. 2009;17:1979–87.
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9.
Marquez-Curtis LA, Gul-Uludag H, Xu P, Chen J, Janowska-Wieczorek A. CXCR4 transfection of cord blood mesenchymal stromal cells with the use of cationic liposome enhances their migration toward stromal cell-derived factor-1. Cytotherapy. 2013;15:840–9.
Sulaiman SB, Chowdhury SR, Busra MFBM, Abdul Rani RB, Mohamad Yahaya NHB, Tabata Y, Hiraoka Y, Haji Idrus RB, Hwei NM. Type II collagen-conjugated mesenchymal stem cells micromass for articular tissue targeting. Biomedicines. 2021;9:880.
Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–10.
Ko IK, Kim B-G, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–72.
Dennis JE, Cohen N, Goldberg VM, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res. 2004;22:735–41.
Xun S. In Situ Articular Cartilage Regeneration through Endogenous Reparative Cell Homing Using a Functional Bone Marrow-Specific Scaffolding System. ACS Appl Mater Interf. 2018;10:38715–28.
Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C, Gao W, Zhou C, Ao Y. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–32.
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, Wang D, Xia J. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–47.
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95.
Cheung CSF, Lui JC, Baron J. Identification of chondrocyte-binding peptides by phage display. J Orthopaedic Res. 2013;31:1053–8.
Cook Sangar ML, Girard EJ, Hopping G, Yin C, Pakiam F, Brusniak MY, Nguyen E, Ruff R, Gewe MM, Byrnes-Blake K, et al. A potent peptide-steroid conjugate accumulates in cartilage and reverses arthritis without evidence of systemic corticosteroid exposure. Sci Transl Med. 2020;12(533):eaay1041.
Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Müller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–8.
Katsumata K, Ishihara J, Fukunaga K, Ishihara A, Yuba E, Budina E, Hubbell JA. Conferring extracellular matrix affinity enhances local therapeutic efficacy of anti-TNF-α antibody in a murine model of rheumatoid arthritis. Arthritis Res Ther. 2019;21:298.
Cuahtecontzi Delint R, Day GJ, Macalester WJP, Kafienah W, Xiao W, Perriman AW. An artificial membrane binding protein-polymer surfactant nanocomplex facilitates stem cell adhesion to the cartilage extracellular matrix. Biomaterials. 2021;276: 120996.
Ren E, Chen H, Qin Z, Guan S, Jiang L, Pang X, He Y, Zhang Y, Gao X, Chu C, et al. Harnessing bifunctional ferritin with kartogenin loading for mesenchymal stem cell capture and enhancing chondrogenesis in cartilage regeneration. Adv Healthcare Mater. 2022;11:2101715.
Vedadghavami A, Wagner EK, Mehta S, He T, Zhang C, Bajpayee AG. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 2019;93:258–69.
Krishnan Y, Rees HA, Rossitto CP, Kim S-E, Hung H-HK, Frank EH, Olsen BD, Liu DR, Hammond PT, Grodzinsky AJ. Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues. Biomaterials. 2018;183:218–33.
Katsumata K, Ishihara J, Mansurov A, Ishihara A, Raczy MM, Yuba E, Hubbell JA. Targeting inflammatory sites through collagen affinity enhances the therapeutic efficacy of anti-inflammatory antibodies. Science Adv. 2019;5:eaay1971–eaay1971.
Ren E, Chen H, Qin Z, Guan S, Jiang L, Pang X, He Y, Zhang Y, Gao X, Chu C, et al. Harnessing bifunctional ferritin with kartogenin loading for mesenchymal stem cell capture and enhancing chondrogenesis in cartilage regeneration. Adv Healthc Mater. 2022;11: e2101715.
Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–54.
Xue S, Zhou X, Sang W, Wang C, Lu H, Xu Y, Zhong Y, Zhu L, He C, Ma J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6:2372–89.
Shortt C, Luyt LG, Turley EA, Cowman MK, Kirsch T. A hyaluronan-binding peptide (P15–1) reduces inflammatory and catabolic events in il-1β-treated human articular chondrocytes. Sci Rep. 2020;10:1441.
Bedingfield SK, Colazo JM, Yu F, Liu DD, Jackson MA, Himmel LE, Cho H, Crofford LJ, Hasty KA, Duvall CL. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat Biomed Eng. 2021;5:1069–83.
Hulme JT, D’Souza WN, McBride HJ, Yoon BP, Willee AM, Duguay A, Thomas M, Fan B, Dayao MR, Rottman JB, et al. Novel protein therapeutic joint retention strategy based on collagen-binding Avimers. J Orthop Res. 2018;36:1238–47.
He T, Zhang C, Vedadghavami A, Mehta S, Clark HA, Porter RM, Bajpayee AG. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Control Rel. 2020;318:109–23.
Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35:538–49.
Silva LHA, Cruz FF, Morales MM, Weiss DJ, Rocco PRM. Magnetic targeting as a strategy to enhance therapeutic effects of mesenchymal stromal cells. Stem Cell Res Ther. 2017;8:58–58.
Huang J, Liang Y, Jia Z, Chen J, Duan L, Liu W, Zhu F, Liang Q, Zhu W, You W, et al. Development of magnetic nanocomposite hydrogel with potential cartilage tissue engineering. ACS Omega. 2018;3:6182–9.
Huang J, Liang Y, Huang Z, Zhao P, Liang Q, Liu Y, Duan L, Liu W, Zhu F, Bian L, et al. Magnetic enhancement of chondrogenic differentiation of mesenchymal stem cells. ACS Biomater Sci Eng. 2019;5:2200–7.
Huang J, Liang Y, Huang Z, Xiong J, Wang D. Preparation, characterization, and biological testing of novel magnetic nanocomposite hydrogels. ACS Omega. 2020;5:9733–43.
Kobayashi T, Ochi M, Yanada S, Ishikawa M, Adachi N, Deie M, Arihiro K. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24:69–76.
Bornes TD, Adesida AB, Jomha NM. Articular cartilage repair with mesenchymal stem cells after chondrogenic priming: a pilot study. Tissue Eng Part A. 2018;24:761–74.
Kamei N, Ochi M, Adachi N, Ishikawa M, Yanada S, Levin LS, Kamei G, Kobayashi T. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2018;26:3626–35.
Qi Y, Yang Z, Ding Q, Zhao T, Huang Z, Feng G. Targeted transplantation of iron oxide-labeled, adipose-derived mesenchymal stem cells in promoting meniscus regeneration following a rabbit massive meniscal defect. Exp Ther Med. 2016;11:458–66.
Go G, Jeong S G, Yoo A, Han J, Kang B, Kim S, Nguyen KT, Jin Z, Kim CS, Seo YR, Kang J Y, Na JY, Song EK, Jeong Y, Seon JK, Park JO, Choi E. Human adipose-derived mesenchymal stem cell-based medical microrobot system for knee cartilage regeneration in vivo. Sci Robot. 2020;5(38):eaay6626.
Li J, Li X, Luo T, Wang R, Liu C, Chen S, Li D, Yue J, Cheng SH, Sun D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci Robot. 2018;3:eaat8829.
Feng Y, Jin X, Dai G, Liu J, Chen J, Yang L. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J Huazhong Univ Sci Technolog Med Sci. 2011;31:204–9.
Mahmoud EE, Kamei G, Harada Y, Shimizu R, Kamei N, Adachi N, Misk NA, Ochi M. Cell magnetic targeting system for repair of severe chronic osteochondral defect in a rabbit model. Cell Transplant. 2016;25:1073–83.
Oshima S, Kamei N, Nakasa T, Yasunaga Y, Ochi M. Enhancement of muscle repair using human mesenchymal stem cells with a magnetic targeting system in a subchronic muscle injury model. J Orthop Sci. 2014;19:478–88.
Kodama A, Kamei N, Kamei G, Kongcharoensombat W, Ohkawa S, Nakabayashi A, Ochi M. In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br. 2012;94:998–1006.
Kamei G, Kobayashi T, Ohkawa S, Kongcharoensombat W, Adachi N, Takazawa K, Shibuya H, Deie M, Hattori K, Goldberg JL, Ochi M. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41:1255–64.
Nedopil A, Klenk C, Kim C, Liu S, Wendland M, Golovko D, Schuster T, Sennino B, McDonald DM, Daldrup-Link HE. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Invest Radiol. 2010;45:634–40.
Kobayashi T, Ochi M, Yanada S, Ishikawa M, Adachi N, Deie M, Arihiro K. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device. Arthroscopy. 2009;25:1435–41.
Acknowledgements
The authors would like to thank Boston Professional Group (BPG) for language editing.
Funding
We would like to acknowledge the financial supported by the Science and Technology Innovation Committee of Shenzhen (No. GJHZ20190820115203714, JSGG20191129094218565, No. JCYJ20200109150700942, GJHZ20200731095608025, GJHZ20130418150248986 and JCYJ20130402101926968), the Guangdong Basic and Applied Basic Research Foundation (2021A1515010985, 2021A1515220134), the Sanming project of medicine in Shenzhen (Grant number SZSM201506020, ZSM202106019).
Author information
Authors and Affiliations
Contributions
YJL conceived and designed the review. JHH and YJL wrote the manuscript. YJL drew schematic figures. QSL revised the manuscript. CX, JYX, LY, and JX contributed to the constructive discussions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors consented to publish this paper.
Competing interests
Authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, J., Liu, Q., Xia, J. et al. Modification of mesenchymal stem cells for cartilage-targeted therapy. J Transl Med 20, 515 (2022). https://doi.org/10.1186/s12967-022-03726-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03726-8