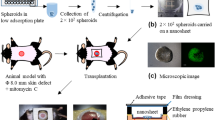
Abstract
BACKGROUND:
Stem cell-based therapies have been developed to treat various types of wounds. Human adipose-derived stem cells (hADSCs) are used to treat skin wounds owing to their outstanding angiogenic potential. Although recent studies have suggested that stem cell spheroids may help wound healing, their cell viability and retention rate in the wound area require improvement to enhance their therapeutic efficacy.
METHODS:
We developed a core–shell structured spheroid with hADSCs in the core and human dermal fibroblasts (hDFs) in the outer part of the spheroid. The core–shell structure was formed by continuous centrifugation and spheroid incubation. After optimizing the method for inducing uniform-sized core–shell spheroids, cell viability, cell proliferation, migration, and therapeutic efficacy were evaluated and compared to those of conventional spheroids.
RESULTS:
Cell proliferation, migration, and involucrin expression were evaluated in keratinocytes. Tubular assays in human umbilical vein endothelial cells were used to confirm the improved skin regeneration and angiogenic efficacy of core–shell spheroids. Core–shell spheroids exhibited exceptional cell viability under hypoxic cell culture conditions that mimicked the microenvironment of the wound area.
CONCLUSION:
The improvement in retention rate, survival rate, and angiogenic growth factors secretion from core–shell spheroids may contribute to the increased therapeutic efficacy of stem cell treatment for skin wounds.






Similar content being viewed by others
References
Cooper DR, Wang C, Patel R, Trujillo A, Patel NA, Prather J, et al. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care (New Rochelle). 2018;7:299–308.
Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20.
Nilforoushzadeh MA, Khodadadi Yazdi M, Baradaran Ghavami S, Farokhimanesh S, Mohammadi Amirabad L, Zarrintaj P, et al. Mesenchymal stem cell spheroids embedded in an injectable thermosensitive hydrogel: an in situ drug formation platform for accelerated wound healing. ACS Biomater Sci Eng. 2020;6:5096–109.
Bhang SH, Lee S, Shin JY, Lee TJ, Kim BS. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138–47.
Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21:1306.
Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176–86.
Lu TY, Yu KF, Kuo SH, Cheng NC, Chuang EY, Yu JS. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers. 2020;12:2997.
Im GB, Kim SW, Bhang SH. Fortifying the angiogenic efficacy of adipose derived stem cell spheroids using spheroid compaction. J Ind Eng Chem. 2021;93:228–36.
Park SJ, Kim KJ, Kim WU, Cho CS. Interaction of mesenchymal stem cells with fibroblast-like synoviocytes via cadherin-11 promotes angiogenesis by enhanced secretion of placental growth factor. J Immunol. 2014;192:3003–10.
Lu W, Yu J, Zhang Y, Ji K, Zhou Y, Li Y, et al. Mixture of fibroblasts and adipose tissue-derived stem cells can improve epidermal morphogenesis of tissue-engineered skin. Cells Tissues Organs. 2012;195:197–206.
Haubner F, Muschter D, Pohl F, Schreml S, Prantl L, Gassner HG. A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. Int J Mol Sci. 2015;16:25947–58.
Zupan J. Mesenchymal stem/stromal cells and fibroblasts: their roles in tissue injury and regeneration, and age-related degeneration. fibroblasts—advances in inflammation. London: IntechOpen 2021; https://www.intechopen.com/chapters/79086https://doi.org/10.5772/intechopen.100556
Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, et al. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials. 2010;31:4855–63.
Singh M, Pierpoint M, Mikos AG, Kasper FK. Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J Biomed Mater Res A. 2011;98:412–24.
Enzerink A, Rantanen V, Vaheri A. Fibroblast nemosis induces angiogenic responses of endothelial cells. Exp Cell Res. 2010;316:826–35.
Chang Y, Li H, Guo Z. Mesenchymal stem cell-like properties in fibroblasts. Cell Physiol Biochem. 2014;34:703–14.
Honnegowda TM, Kumar P, Udupa EGP, Kumar S, Kumar U, Rao P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthet Res. 2015;2:243–9.
Uitterdijk A, Groenendijk BC, Gorsse-Bakker C, Panasewicz A, Sneep S, Tempel D, et al. Time course of VCAM-1 expression in reperfused myocardial infarction in swine and its relation to retention of intracoronary administered bone marrow-derived mononuclear cells. PLoS One. 2017;12:e0178779.
Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18:240–9.
Jo H, Gajendiran M, Kim K. Influence of PEG chain length on colloidal stability of mPEGylated polycation based coacersomes for therapeutic protein delivery. J Ind Eng Chem. 2020;82:234–42.
Murphy KC, Hung BP, Browne-Bourne S, Zhou D, Yeung J, Genetos DC, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017;14:20160851.
Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci. 2007;12:4722–30.
Loo DT. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. In: Didenko V. Editor. DNA Damage Detection In Situ, Ex Vivo, and In Vivo. Methods in Molecular Biology, 2011; Vol 682. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-409-8_1
Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–44.
Yu F, Lin Y, Zhan T, Chen L, Guo S. HGF expression induced by HIF-1α promote the proliferation and tube formation of endothelial progenitor cells. Cell Biol Int. 2015;39:310–7.
Santos JM, Camões SP, Filipe E, Cipriano M, Barcia RN, Filipe M, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6:90.
Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–90.
Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Func Mater. 2015;25:1344–51.
Kim SJ, Park J, Byun H, Park YW, Major LG, Lee DY, et al. Hydrogels with an embossed surface: an all-in-one platform for mass production and culture of human adipose-derived stem cell spheroids. Biomaterials. 2019;188:198–212.
Jauković A, Abadjieva D, Trivanović D, Stoyanova E, Kostadinova M, Pashova S, et al. Specificity of 3D MSC spheroids microenvironment: impact on MSC behavior and properties. Stem Cell Rev Rep. 2020;16:853–75.
Wang D, Shen W, Zhang F, Chen M, Chen H, Cao K. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int. 2010;34:415–23.
Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song B, et al. High-efficient generation of VCAM-1+ mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020;53:e12862.
Aomatsu E, Chosa N, Nishihira S, Sugiyama Y, Miura H, Ishisaki A. Cell-cell adhesion through N-cadherin enhances VCAM-1 expression via PDGFRβ in a ligand-independent manner in mesenchymal stem cells. Int J Mol Med. 2014;33:565–72.
Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:3706315.
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D, et al. Hypoxia-inducible factor 1 alpha–activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54:910–9.
Qu Y, Cao C, Wu Q, Huang A, Song Y, Li H, et al. The dual delivery of KGF and b FGF by collagen membrane to promote skin wound healing. J Tissue Eng Regen Med. 2018;12:1508–18.
Kisling A, Lust RM, Katwa LC. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–4.
Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–95.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), and the Ministry of Science and ICT (NRF-2018M3A9E2023255, NRF-2019R1C1C1007384, NRF-2020M2D9A3094171, and NRF-2021R1A4A1032782). This research was also supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare; project Number: 21A0102L1-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial conflicts of interest.
Ethical statement
No experiments involving animals or humans were conducted in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, DH., Bhang, S.H. Development of Hetero-Cell Type Spheroids Via Core–Shell Strategy for Enhanced Wound Healing Effect of Human Adipose-Derived Stem Cells. Tissue Eng Regen Med 20, 581–591 (2023). https://doi.org/10.1007/s13770-022-00512-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00512-x