Abstract
Background:
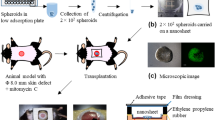
Current in vivo adult stem cell delivery presents limited clinical effects due to poor engraftment and survival. To overcome current challenges in cell delivery and promote surgical cell delivery for soft tissue repair, a multi-spheroid-loaded thin sectioned acellular dermal matrix (tsADM) carrier which preserves loaded spheroids’ three-dimensional (3D) structure, was developed.
Methods:
Adipose-derived stem cells (ASCs) were used for spheroid delivery. After generating spheroids in 3D cell culture dishes, spheroid plasticity and survival in-between coverslips were evaluated. Spheroids were loaded onto tsADM, their shape changes were followed up for 14 days, and then imaged. Spheroid adhesion stability to tsADM against shear stress was also evaluated. Finally, cell delivery efficacy was compared with cell-seeded tsADM by in vivo implantation and histological evaluation.
Results:
Spheroids withstood cyclic compression stress and maintained their 3D shape without fusion after 48 h of culture in-between coverslips. Cell survival improved when spheroids were cultured on tsADM in-between the coverslips. Spheroid-loaded tsADM with coverslips maintained their spheroid outline for 14 days of culture whereas without coverslips, the group lost their outline due to spreading after 4 days in culture. Spheroids loaded onto tsADMs were more stable after six rather than 3 days in culture. Spheroid-loaded tsADMs showed about a 2.96-fold higher ASCs transplantation efficacy than cell-seeded tsADMs after 2 weeks of in vivo transplantation.
Conclusion:
These results indicate that transplantation of spheroid-loaded tsADMs significantly improved cell delivery. These findings suggest that a combined approach with other cells, drugs, and nanoparticles may improve cell delivery and therapeutic efficacy.










Similar content being viewed by others
Change history
28 July 2020
Unfortunately, the online published article has error in figure��2 caption.
References
Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–7.
Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–91.
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70.
Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–4.
Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9.
Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–22.
Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114:e107–16.
Wu KH, Mo XM, Han ZC, Zhou B. Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg. 2011;92:1917–25.
Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–74.
Voegele TJ, Voegele-Kadletz M, Esposito V, Macfelda K, Oberndorfer U, Vecsei V, et al. The effect of different isolation techniques on human osteoblast-like cell growth. Anticancer Res. 2000;20:3575–81.
Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–15.
Mendez I, Hong M, Smith S, Dagher A, Desrosiers J. Neural transplantation cannula and microinjector system: experimental and clinical experience. J Neurosurg. 2000;92:493–9.
Altman A, Matthias N, Yan Y, Song YH, Bai X, Chiu E, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29:1431–42.
Gilmore AP. Anoikis. Cell Death Differ. 2005;12:S1473–7.
Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–32.
Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1:382–93.
Orbay H, Takami Y, Hyakusoku H, Mizuno H. Acellular dermal matrix seeded with adipose-derived stem cells as a subcutaneous implant. Aesthetic Plast Surg. 2011;35:756–63.
Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–6.
Ducheyne P, Healy K, Hutmacher DW, Grainger DW, Kirkpatrick CJ. Comprehensive biomaterials. Amsterdam: Elsevier; 2015.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
Moku G, Layek B, Trautman L, Putnam S, Panyam J, Prabha S. Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers (Basel). 2019;11:E491.
Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8.
Doornaert M, Depypere B, Creytens D, Declercq H, Taminau J, Lemeire K, et al. Human decellularized dermal matrix seeded with adipose-derived stem cells enhances wound healing in a murine model: Experimental study. Ann Med Surg (Lond). 2019;46:4–11.
Garg A, Houlihan DD, Aldridge V, Suresh S, Li KK, King AL, et al. Non-enzymatic dissociation of human mesenchymal stromal cells improves chemokine-dependent migration and maintains immunosuppressive function. Cytotherapy. 2014;16:545–59.
Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993;27:1243–51.
Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–80.
Cheng S, Robbins MO. Nanocapillary adhesion between parallel plates. Langmuir. 2016;32:7788–95.
Agrawal H, Shang H, Sattah AP, Yang N, Peirce SM, Katz AJ. Human adipose-derived stromal/stem cells demonstrate short-lived persistence after implantation in both an immunocompetent and an immunocompromised murine model. Stem Cell Res Ther. 2014;5:142.
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204.
Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–47.
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–24.
Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715.
von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–2.
Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–9.
Ní Annaidh A, Bruyère K, Destrade M, Gilchrist MD, Maurini C, Otténio M, et al. Automated estimation of collagen fibre dispersion in the dermis and its contribution to the anisotropic behaviour of skin. Ann Biomed Eng. 2012;40:1666–78.
Hong JK, Yun J, Kim H, Kwon SM. Three-dimensional culture of mesenchymal stem cells. Tissue Eng Regen Med. 2015;12:211–21.
Jeon JH, Yun BG, Lim MJ, Kim SJ, Lim MH, Lim JY, et al. Rapid cartilage regeneration of spheroids composed of human nasal septum-derived chondrocyte in rat osteochondral defect model. Tissue Eng Regen Med. 2020;17:81–90.
Park IS, Chung PS, Ahn JC. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS One. 2015;10:e0122776.
Cheng NC, Chen SY, Li JR, Young TH. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med. 2013;2:584–94.
Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–58.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1C1B5017905). This research was supported by a Grant of Translational R&D Project through Institute for Bio-Medical convergence, Incheon St. Mary’s Hospital, The Catholic University of Korea
Author information
Authors and Affiliations
Contributions
JHK performed the experiments, analyzed the data and co-wrote the paper. JYL conceived of the presented idea, designed the study, performed the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical statement
All in vivo experiments were carried out in accordance with the Institutional Animal Care and Use Committee (IACUC) of Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (IACUC Approval No. 2019-008).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.H., Lee, J.Y. Multi-Spheroid-Loaded Human Acellular Dermal Matrix Carrier Preserves Its Spheroid Shape and Improves In Vivo Adipose-Derived Stem Cell Delivery and Engraftment. Tissue Eng Regen Med 17, 271–283 (2020). https://doi.org/10.1007/s13770-020-00252-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00252-w